Chemistry:Enprostil
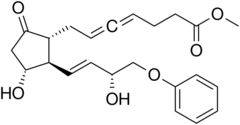 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C23H28O6 |
| Molar mass | 400.471 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Enprostil is a synthetic prostaglandin designed to resemble dinoprostone. Enprostil was found to be a highly potent inhibitor of gastric HCl secretion.[1] It is an analog of prostaglandin E2 but unlike this prostaglandin, which binds to and activates all four cellular receptors viz., EP1, EP2, EP3, and EP4 receptors, enprostil is a more selective receptor agonist in that it binds to and activates primarily the EP3 receptor.[2] Consequently, enprostil is expected to have a narrower range of actions that may avoid some of the unwanted side-effects and toxicities of prostaglandin E2. A prospective multicenter randomized controlled trial conducted in Japan found combining enprostil with cimetidine was more effective than cimetidine alone in treating gastric ulcer.[3]
See also
- Prostaglandin receptors
- EP3 and peptic ulcer
References
- ↑ "Gastric antisecretory and antiulcer properties of enprostil, (+/-)-11 alpha, 15 alpha-dihydroxy-16-phenoxy-17,18,19,20-tetranor-9-oxoprosta- 4,5,13(t)-trienoic acid methyl ester". The Journal of Pharmacology and Experimental Therapeutics 239 (2): 382–389. November 1986. PMID 3095537.
- ↑ "Eicosanoid receptors: Targets for the treatment of disrupted intestinal epithelial homeostasis". European Journal of Pharmacology 796: 7–19. February 2017. doi:10.1016/j.ejphar.2016.12.004. PMID 27940058.
- ↑ "Combination of enprostil and cimetidine is more effective than cimetidine alone in treating gastric ulcer: prospective multicenter randomized controlled trial". Hepato-Gastroenterology 52 (66): 1925–1929. 2005. PMID 16334808.
Further reading
- "Enprostil, a prostaglandin-E(2) analogue, inhibits interleukin-8 production of human colonic epithelial cell lines". Scandinavian Journal of Immunology 52 (6): 570–575. December 2000. doi:10.1046/j.1365-3083.2000.00815.x. PMID 11119262.
- "Effect of enprostil on omeprazole-induced hypergastrinemia and inhibition of gastric acid secretion in peptic ulcer patients". Digestive Diseases and Sciences 42 (8): 1741–1746. August 1997. doi:10.1023/A:1018825902055. PMID 9286243.
- "A comparison of two prostaglandin analogues (enprostil vs misoprostol) in the treatment of acute duodenal ulcer disease". Journal of Gastroenterology 30 (5): 607–614. October 1995. doi:10.1007/BF02367786. PMID 8574332.
 |

