Chemistry:Abecarnil
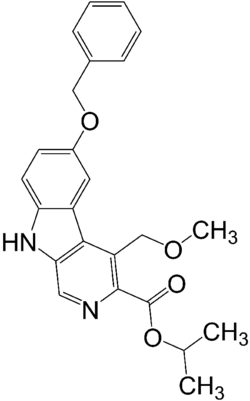 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Pharmacokinetic data | |
| Elimination half-life | 3.4 hours (IV), 7 hours (oral) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C24H24N2O4 |
| Molar mass | 404.466 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Abecarnil (ZK-112,119) is an anxiolytic drug from the β-Carboline family. It is one of a relatively recently developed class of medicines known as the nonbenzodiazepines, which have similar effects to the older benzodiazepine group, but with quite different chemical structures. It is a partial agonist acting selectively at the benzodiazepine site of the GABAA receptor.[1]
Abecarnil was originally developed as an anti-anxiety drug, but has not as yet been commercially developed for use in humans, instead so far mainly being used for research into the development of other new sedative and anxiolytic drugs. Investigations are continuing into its actions and it looks likely to be developed for use both in the treatment of anxiety,[2] and as a less addictive substitute drug for the treatment of benzodiazepine[3] and alcohol[4] addiction. Abecarnil may also have fewer problems of tolerance and withdrawal problems compared to nonselective full agonist benzodiazepine acting drugs.[5]
Abecarnil is a relatively subtype-selective drug which produces primarily anxiolytic effects, with comparatively less sedative or muscle relaxant properties,[6][7] and does not significantly potentiate the effects of alcohol.[8]
The abuse potential of abecarnil is thought to be less than that of benzodiazepines,[9] with only mild withdrawal symptoms noted after abrupt discontinuation of treatment.[10]
See also
References
- ↑ "Pharmacological characterization of the novel anxiolytic beta-carboline abecarnil in rodents and primates". Japanese Journal of Pharmacology 64 (3): 179–187. March 1994. doi:10.1254/jjp.64.179. PMID 7912751.
- ↑ "Abecarnil, a new beta-carboline, in the treatment of anxiety disorders". The British Journal of Psychiatry. Supplement 34 (34): 55–63. 1998. doi:10.1192/S0007125000293537. PMID 9829018.
- ↑ "Alprazolam dependence prevented by substituting with the beta-carboline abecarnil". Proceedings of the National Academy of Sciences of the United States of America 94 (6): 2719–2723. March 1997. doi:10.1073/pnas.94.6.2719. PMID 9122263. Bibcode: 1997PNAS...94.2719P.
- ↑ "Abecarnil and alprazolam reverse anxiety-like behaviors induced by ethanol withdrawal". Alcohol 21 (2): 161–168. June 2000. doi:10.1016/S0741-8329(00)00079-3. PMID 10963939.
- ↑ "Withdrawal precipitation by benzodiazepine receptor antagonists in dogs chronically treated with diazepam or the novel anxiolytic and anticonvulsant beta-carboline abecarnil". Naunyn-Schmiedeberg's Archives of Pharmacology 345 (4): 452–460. April 1992. doi:10.1007/BF00176624. PMID 1352384.
- ↑ "Pharmacokinetics and acute toleration of the beta-carboline derivative abecarnil in man". Arzneimittel-Forschung 40 (5): 529–532. May 1990. PMID 1974428.
- ↑ "Human studies on abecarnil a new beta-carboline anxiolytic: safety, tolerability and preliminary pharmacological profile". British Journal of Clinical Pharmacology 35 (4): 386–394. April 1993. doi:10.1111/j.1365-2125.1993.tb04155.x. PMID 8097921.
- ↑ "Abecarnil, a metabolically stable, anxioselective beta-carboline acting at benzodiazepine receptors". The Journal of Pharmacology and Experimental Therapeutics 253 (1): 334–343. April 1990. PMID 1970361.
- ↑ "Behavioral pharmacology of abecarnil in baboons: self-injection, drug discrimination and physical dependence". Behavioural Pharmacology 3 (5): 507–516. October 1992. doi:10.1097/00008877-199210000-00009. PMID 11224153.
- ↑ "The first double-blind, placebo-controlled trial of a partial benzodiazepine agonist abecarnil (ZK 112-119) in generalized anxiety disorder". Psychopharmacology Bulletin 27 (2): 171–179. 1991. PMID 1681563.
 |
