Chemistry:Nilutamide
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | nye-LOO-tah-mide[1] |
| Trade names | Nilandron, Anandron |
| Other names | RU-23908 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697044 |
| Pregnancy category |
|
| Routes of administration | By mouth[3] |
| Drug class | Nonsteroidal antiandrogen |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Good[3] |
| Protein binding | 80–84%[4] |
| Metabolism | Liver (CYP2C19, FMO)[3][4] |
| Metabolites | At least 5, some active[4][5] |
| Elimination half-life | Mean: 56 hours (~2 days)[6] Range: 23–87 hours[6] |
| Excretion | Urine: 62%[3][4] Feces: <10%[3][4] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
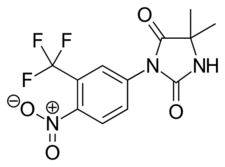
| Formula | C12H10F3N3O4 |
| Molar mass | 317.224 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 149 °C (300 °F) |
| |
| |
| (verify) | |
Nilutamide, sold under the brand names Nilandron and Anandron, is a nonsteroidal antiandrogen (NSAA) which is used in the treatment of prostate cancer.[7][8][9][10][11][12] It has also been studied as a component of feminizing hormone therapy for transgender women and to treat acne and seborrhea in women.[13][14][15][16] It is taken by mouth.[4]
Side effects in men include breast tenderness and enlargement, feminization, sexual dysfunction, and hot flashes.[17][18][19][20] Nausea, vomiting, visual disturbances, alcohol intolerance, elevated liver enzymes, and lung disease can occur in both sexes.[20][21][18][22][23][24] Rarely, nilutamide can cause respiratory failure and liver damage.[17][20] These unfavorable side effects, along with a number of associated cases of death, have limited the use of nilutamide.[12][25][26]
Nilutamide acts as a selective antagonist of the androgen receptor (AR), preventing the effects of androgens like testosterone and dihydrotestosterone (DHT) in the body.[27][13] Because most prostate cancer cells rely on these hormones for growth and survival, nilutamide can slow the progression of prostate cancer and extend life in men with the disease.[13]
Nilutamide was discovered in 1977 and was first introduced for medical use in 1987.[8][28][29][6] It became available in the United States in 1996.[30][31][32] The drug has largely been replaced by newer and improved NSAAs, namely bicalutamide and enzalutamide, due to their better efficacy, tolerability, and safety, and is now rarely used.[33]
It is on the World Health Organization's List of Essential Medicines.[34]
Medical uses
Prostate cancer
Nilutamide is used in prostate cancer in combination with a gonadotropin-releasing hormone (GnRH) analogue at a dosage of 300 mg/day (150 mg twice daily) for the first 4 weeks of treatment, and 150 mg/day thereafter.[26][35] It is not indicated as a monotherapy in prostate cancer.[26] Only one small non-comparative study has assessed nilutamide as a monotherapy in prostate cancer.[36]
Nilutamide has been used to prevent the effects of the testosterone flare at the start of GnRH agonist therapy in men with prostate cancer.[37][38][39]
Transgender hormone therapy
Nilutamide has been studied for use as a component of feminizing hormone therapy for transgender women.[13][14] It has been assessed in at least five small clinical studies for this purpose in treatment-naive subjects.[14][40][41][42][43][44] In these studies, nilutamide monotherapy at a dosage of 300 mg/day, induced observable signs of clinical feminization in young transgender women (age range 19–33 years) within 8 weeks,[41] including breast development, decreased body hair (though not facial hair),[40] decreased morning erections and sex drive,[42] and positive psychological and emotional changes.[42][45] Signs of breast development occurred in all subjects within 6 weeks and were associated with increased nipple sensitivity,[44][41][42] and along with decreased hair growth, were the earliest sign of feminization.[41]
Nilutamide did not change the size of the prostate gland (which is the same as with high-dosage cyproterone acetate and ethinylestradiol treatment for as long as 18 months), but was found to alter its histology, including increased stromal tissue with a significant reduction in acini and atrophic epithelial cells, indicating glandular atrophy.[43][44][46] In addition, readily apparent histological changes were observed in the testes, including a reduction in tubular and interstitial cells.[43]
Nilutamide was found to more than double luteinizing hormone (LH) and testosterone levels and to triple estradiol levels.[40][41][43] In contrast, follicle-stimulating hormone levels remained unchanged.[41][43] A slight but significant increase in prolactin levels was observed, and levels of sex hormone-binding globulin increased as well.[41][43] The addition of ethinylestradiol to nilutamide therapy after 8 weeks abolished the increase in LH, testosterone, and estradiol levels and dramatically suppressed testosterone levels, into the castrate range.[40][41] Both nilutamide alone and the combination of nilutamide and estrogen were regarded as resulting in effective and favorable antiandrogen action and feminization in transgender women.[40][41]
Skin conditions
Nilutamide has been assessed in the treatment of acne and seborrhea in women in at least one small clinical study.[15][16] The dosage used was 200 mg/day, and in the study, "seborrhea and acne decreased markedly within the first month and practically disappeared after 2 months of [nilutamide] treatment."[15][16]
Available forms
Nilutamide is available in the form of 50 and 150 mg oral tablets.[47]
Side effects
General side effects of NSAAs, including nilutamide, include gynecomastia, breast pain/tenderness, hot flashes (67%), depression, fatigue, sexual dysfunction (including loss of libido and erectile dysfunction), decreased muscle mass, and decreased bone mass with an associated increase in fractures.[18][19][20] Also, nausea (24–27%), vomiting, constipation (20%), and insomnia (16%) may occur with nilutamide.[20] Nilutamide monotherapy is known to eventually induce gynecomastia in 40 to 80% of men treated with it for prostate cancer, usually within 6 to 9 months of treatment initiation.[48][49][50][51]
Relative to other NSAAs, nilutamide has been uniquely associated with mild and reversible visual disturbances (31–58%) including delayed ocular adaptation to darkness and impaired color vision,[21] a disulfiram-like[18] alcohol intolerance (19%), interstitial pneumonitis (0.77–2.4%)[33][52][53] (which can result in dyspnea (1%) as a secondary effect and can progress to pulmonary fibrosis),[22] and hepatitis (1%), and has a higher incidence of nausea and vomiting compared to other NSAAs.[12][26][20][54] The incidence of interstitial pneumonitis with nilutamide has been found to be much higher in Japanese patients (12.6%), warranting particular caution in Asian individuals.[55][56] There is a case report of simultaneous liver and lung toxicity in a nilutamide-treated patient.[57]
There is also a risk of hepatotoxicity with nilutamide, though occurrence is very rare and the risk is significantly less than with flutamide.[6][58] The incidence of abnormal liver function tests (e.g., elevated liver enzymes) has been variously reported as 2 to 33% with nilutamide.[59][1] For comparison, the risk of elevated liver enzymes has been reported as 4 to 62% in the case of flutamide.[59][23][6] The risk of hepatotoxicity with nilutamide has been described as far less than with flutamide.[1] Fulminant hepatic failure has been reported for nilutamide, with fatal outcome.[6][60][61][62] Between 1986 and 2003, the numbers of published cases of hepatotoxicity for antiandrogens totaled 46 for flutamide, 21 for cyproterone acetate, 4 for nilutamide, and 1 for bicalutamide.[63] Similarly to flutamide, nilutamide exhibits mitochondrial toxicity in hepatocytes by inhibiting respiratory complex I (NADH ubiquinone oxidoreductase) (though not respiratory complexes II, III, or IV) in the electron transport chain, resulting in reduced ATP and glutathione production and thus decreased hepatocyte survival.[62][64][65] The nitro group of nilutamide has been theorized to be involved in both its hepatotoxicity and its pulmonary toxicity.[65][66]
Pharmacology
Pharmacodynamics
Antiandrogenic activity
Nilutamide acts as a selective competitive silent antagonist of the AR (IC50 = 412 nM),[27] which prevents androgens like testosterone and DHT from activating the receptor.[13] The affinity of nilutamide for the AR is about 1 to 4% of that of testosterone and is similar to that of bicalutamide and 2-hydroxyflutamide.[67][68][69] Similarly to 2-hydroxyflutamide, but unlike bicalutamide, nilutamide is able to weakly activate the AR at high concentrations.[68] It does not inhibit 5α-reductase.[70]
Like other NSAAs such as flutamide and bicalutamide, nilutamide, without concomitant GnRH analogue therapy, increases serum androgen (by two-fold in the case of testosterone), estrogen, and prolactin levels due to inhibition of AR-mediated suppression of steroidogenesis via negative feedback on the hypothalamic–pituitary–gonadal axis.[13] As such, though nilutamide is still effective as an antiandrogen as a monotherapy, it is given in combination with a GnRH analogue such as leuprorelin in prostate cancer to suppress androgen concentrations to castrate levels in order to attain maximal androgen blockade (MAB).[13]
Like flutamide and bicalutamide, nilutamide is able to cross the blood–brain barrier and has central antiandrogen actions.[71]
Cytochrome P450 inhibition
Nilutamide is known to inhibit several cytochrome P450 enzymes, including CYP1A2, CYP2C9, and CYP3A4, and can result in increased levels of medications that are metabolized by these enzymes.[72] It has also been found to inhibit the enzyme CYP17A1 (17α-hydroxylase/17,20-lyase) in vitro and thus the biosynthesis of androgens.[73][74] However, nilutamide monotherapy significantly increases testosterone levels in vivo, so the clinical significance of this finding is uncertain.[73][74]
Pharmacokinetics
Nilutamide has an elimination half-life of 23 to 87 hours, with a mean of 56 hours,[6] or about two days; this allows for once-daily administration.[12] Steady state (plateau) levels of the drug are attained after two weeks of administration with a dosage of 150 mg twice daily (300 mg/day total).[75] It is metabolized by CYP2C19, with at least five metabolites.[5] Virtually all of the antiandrogenic activity of nilutamide comes from the parent drug (as opposed to metabolites).[76]
Chemistry
Nilutamide is structurally related to the first-generation NSAAs flutamide and bicalutamide as well as to the second-generation NSAAs enzalutamide and apalutamide.
History
Nilutamide was developed by Roussel and was first described in 1977.[8][28][29] It was first introduced for medical use in 1987 in France [6][77] and was the second NSAA to be marketed, with flutamide preceding it and bicalutamide following it in 1995.[12][78] It was not introduced until 1996 in the United States .[30][31][32]
Society and culture
Generic names
Nilutamide is the generic name of the drug and its INN, USAN, BAN, and DCF.[8][9][10][11]
Brand names
Nilutamide is marketed under the brand name Nilandron in the United States and under the brand name Anandron elsewhere in the world such as in Australia , Canada , Europe, and Latin America.[9][11]
Availability
Nilutamide is or has been available in the United States, Canada, Australia, Europe, Latin America, Egypt, and Lebanon.[9][11] In Europe, it is or has been available in Belgium, Croatia, the Czech Republic, Finland , France , the Netherlands, Norway , Poland , Portugal, Serbia, Sweden, Switzerland , and Yugoslavia.[9][11] in Latin America, it is or has been available in Argentina , Brazil , and Mexico.[9][11]
Research
The combination of an estrogen and nilutamide as a form of combined androgen blockade for the treatment of prostate cancer has been studied in animals.[79]
Nilutamide has been studied in the treatment of advanced breast cancer.[80][81]
References
- ↑ 1.0 1.1 1.2 "Nilutamide - LiverTox". https://livertox.nih.gov/Nilutamide.htm. "In large registration clinical trials, ALT elevations occurred in 2% to 33% of patients during nilutamide therapy. The elevations were usually mild, asymptomatic and transient, rarely requiring drug discontinuation. In rare instances, clinically apparent acute liver injury has occurred during nilutamide therapy, but the number of published cases are few, and the agent appears to be far less hepatotoxic than flutamide."
- ↑ "Nilutamide (Nilandron) Use During Pregnancy". https://www.drugs.com/pregnancy/nilutamide.html.
- ↑ 3.0 3.1 3.2 3.3 3.4 Perry's The Chemotherapy Source Book. Lippincott Williams & Wilkins. 30 July 2012. pp. 711–. ISBN 978-1-4698-0343-2. https://books.google.com/books?id=My3SjQTguyYC&pg=PA711.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. 24 January 2012. pp. 1373–. ISBN 978-1-60913-345-0. https://books.google.com/books?id=Sd6ot9ul-bUC&pg=PA1373.
- ↑ 5.0 5.1 Cancer Chemotherapy and Biotherapy: Principles and Practice. Lippincott Williams & Wilkins. 8 November 2010. pp. 680–. ISBN 978-1-60547-431-1. https://books.google.com/books?id=WL4arNFsQa8C&pg=PA680.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 "Nonsteroidal Antiandrogens". Hormone Therapy in Breast and Prostate Cancer. Humana Press. 2009. pp. 347–368. doi:10.1007/978-1-59259-152-7_16. ISBN 978-1-60761-471-5. "Although the t1/2 of nilutamide is h (mean 56 h) (39), suggesting that once-daily dosing would be appropriate, a three times per day regimen has been employed in most clinical trials."
- ↑ https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020169s008lbl.pdf [bare URL PDF]
- ↑ 8.0 8.1 8.2 8.3 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 873–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA873.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 737–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA737.
- ↑ 10.0 10.1 Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 199–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA199.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 "Nilutamide". https://www.drugs.com/international/nilutamide.html.
- ↑ 12.0 12.1 12.2 12.3 12.4 Textbook of Prostate Cancer: Pathology, Diagnosis and Treatment: Pathology, Diagnosis and Treatment. CRC Press. 1 March 1999. pp. 280–. ISBN 978-1-85317-422-3. https://books.google.com/books?id=GreZlojD-tYC&pg=PA280.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 Antiandrogens in Prostate Cancer: A Key to Tailored Endocrine Treatment. Springer Science & Business Media. 6 December 2012. pp. 194–210. ISBN 978-3-642-45745-6. https://books.google.com/books?id=jqZDBQAAQBAJ&pg=PT194.
- ↑ 14.0 14.1 14.2 Gender Dysphoria and Disorders of Sex Development: Progress in Care and Knowledge. Springer Science & Business Media. 1 July 2013. pp. 280–. ISBN 978-1-4614-7441-8. https://books.google.com/books?id=YQ5GAAAAQBAJ&pg=PA280.
- ↑ 15.0 15.1 15.2 "Effects of a pure antiandrogen on gonadotropin secretion in normal women and in polycystic ovarian disease". Fertility and Sterility 52 (1): 42–50. July 1989. doi:10.1016/s0015-0282(16)60786-0. PMID 2744186.
- ↑ 16.0 16.1 16.2 "Clinical applications of antiandrogens". Journal of Steroid Biochemistry 31 (4B): 719–729. October 1988. doi:10.1016/0022-4731(88)90023-4. PMID 2462132.
- ↑ 17.0 17.1 "Nilutamide: an antiandrogen for the treatment of prostate cancer". The Annals of Pharmacotherapy 31 (1): 65–75. January 1997. doi:10.1177/106002809703100112. PMID 8997470.
- ↑ 18.0 18.1 18.2 18.3 Medical Toxicology. Lippincott Williams & Wilkins. 2004. pp. 521–. ISBN 978-0-7817-2845-4. https://books.google.com/books?id=BfdighlyGiwC&pg=PA521.
- ↑ 19.0 19.1 Neurologic Complications of Cancer. Oxford University Press, USA. 12 September 2008. pp. 479–. ISBN 978-0-19-971055-3. https://books.google.com/books?id=mpZ8Dp2KdHMC&pg=PA479.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 Pharmacology for Nursing Care. Elsevier Health Sciences. 2013. pp. 1297–. ISBN 978-1-4377-3582-6. https://books.google.com/books?id=_4SwO2dHcAIC&pg=PA1297.
- ↑ 21.0 21.1 Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. 2001. pp. 1196–. ISBN 978-0-7817-1750-2. https://books.google.com/books?id=FVfzRvaucq8C&pg=PA1196.
- ↑ 22.0 22.1 Campbell-Walsh Urology: Expert Consult Premium Edition: Enhanced Online Features and Print, 4-Volume Set. Elsevier Health Sciences. 25 August 2011. pp. 2939–. ISBN 978-1-4160-6911-9. https://books.google.com/books?id=fu3BBwAAQBAJ&pg=PA2939.
- ↑ 23.0 23.1 "Bicalutamide-induced hepatotoxicity: A rare adverse effect". The American Journal of Case Reports 15: 266–270. 2014. doi:10.12659/AJCR.890679. PMID 24967002.
- ↑ Pharmacology for Pharmacy and the Health Sciences: A Patient-centred Approach. OUP Oxford. 25 March 2010. pp. 632–. ISBN 978-0-19-955982-4. https://books.google.com/books?id=KVicAQAAQBAJ&pg=PA632.
- ↑ Prostate and Other Genitourinary Cancers: Cancer: Principles & Practice of Oncology. Wolters Kluwer Health. 18 March 2016. pp. 1006–. ISBN 978-1-4963-5421-1. https://books.google.com/books?id=Bf3DCwAAQBAJ&pg=PT1006.
- ↑ 26.0 26.1 26.2 26.3 Prostate Cancer: Basic Mechanisms and Therapeutic Approaches. World Scientific. 1 January 2005. pp. 11–. ISBN 978-981-256-920-2. https://books.google.com/books?id=4xQAr3Uh8EUC&pg=PA11.
- ↑ 27.0 27.1 "Androgen receptor antagonists (antiandrogens): structure-activity relationships". Current Medicinal Chemistry 7 (2): 211–247. February 2000. doi:10.2174/0929867003375371. PMID 10637363.
- ↑ 28.0 28.1 "Interactions Between LHRH, Sex Steroids and "Inhibin" in the Control of LH and FSH Secretion". International Journal of Andrology 1 (s2a): 81–101. 1978. doi:10.1111/j.1365-2605.1978.tb00008.x. ISSN 0105-6263.
- ↑ 29.0 29.1 "Action of a non-steroid anti-androgen, RU 23908, in peripheral and central tissues". Journal of Steroid Biochemistry 11 (1A): 93–99. July 1979. doi:10.1016/0022-4731(79)90281-4. PMID 385986.
- ↑ 30.0 30.1 Estrogens, Progestins, and Their Antagonists: Health Issues. Springer Science & Business Media. 6 December 2012. pp. 167–. ISBN 978-1-4612-4096-9. https://books.google.com/books?id=LAnpBwAAQBAJ&pg=PA167.
- ↑ 31.0 31.1 "Structural basis for antagonism and resistance of bicalutamide in prostate cancer". Proceedings of the National Academy of Sciences of the United States of America 102 (17): 6201–6206. April 2005. doi:10.1073/pnas.0500381102. PMID 15833816. Bibcode: 2005PNAS..102.6201B.
- ↑ 32.0 32.1 "Nilutamide - AdisInsight". http://adisinsight.springer.com/drugs/800004379.
- ↑ 33.0 33.1 Prostate Cancer. Demos Medical Publishing. 2011. pp. 81–. ISBN 978-1-935281-91-7. https://books.google.com/books?id=WJkjgbRJe3EC&pg=PT81.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ The Australian Drug Guide: Every Person's Guide to Prescription and Over-the-counter Medicines, Street Drugs, Vaccines, Vitamins and Minerals.... Black Inc.. 2006. pp. 283–. ISBN 978-1-86395-174-6. https://books.google.com/books?id=7O9kiqN2q2YC&pg=PA283.
- ↑ "The role of antiandrogen monotherapy in the treatment of prostate cancer". BJU International 91 (5): 455–461. March 2003. doi:10.1046/j.1464-410X.2003.04026.x. PMID 12603397. "Trial experience with nilutamide monotherapy is limited to one small non-comparative study involving 26 patients with metastatic disease given nilutamide 100 mg three times daily (the dose used when nilutamide is administered as a component of MAB) [14]. The median progression-free survival in these patients was 9 months, with a median overall survival of 23 months. There have been no comparative trials of nilutamide with other antiandrogens or with castration [15]. The limited available data on nilutamide monotherapy means that no meaningful conclusions about the role of nilutamide in this setting can be determined. Nilutamide is not licensed as monotherapy.".
- ↑ "Flare Associated with LHRH-Agonist Therapy". Reviews in Urology 3 (Suppl 3): S10–S14. 2001. PMID 16986003.
- ↑ "Disease flare with gonadotrophin-releasing hormone (GnRH) analogues. How serious is it?". Drug Safety 8 (4): 265–270. April 1993. doi:10.2165/00002018-199308040-00001. PMID 8481213.
- ↑ "Prevention of the transient adverse effects of a gonadotropin-releasing hormone analogue (buserelin) in metastatic prostatic carcinoma by administration of an antiandrogen (nilutamide)". The New England Journal of Medicine 321 (7): 413–418. August 1989. doi:10.1056/NEJM198908173210701. PMID 2503723.
- ↑ 40.0 40.1 40.2 40.3 40.4 "Reduction in undesired sexual hair growth with anandron in male-to-female transsexuals--experiences with a novel androgen receptor blocker". Clinical and Experimental Dermatology 14 (5): 361–363. September 1989. doi:10.1111/j.1365-2230.1989.tb02585.x. PMID 2612040.
- ↑ 41.0 41.1 41.2 41.3 41.4 41.5 41.6 41.7 41.8 "Merits and considerations in the use of anti-androgen". Journal of Steroid Biochemistry 31 (4B): 731–737. October 1988. doi:10.1016/0022-4731(88)90024-6. PMID 3143862.
- ↑ 42.0 42.1 42.2 42.3 "Effects of the pure antiandrogen RU 23.903 (anandron) on sexuality, aggression, and mood in male-to-female transsexuals". Archives of Sexual Behavior 18 (3): 217–228. June 1989. doi:10.1007/BF01543196. PMID 2751416.
- ↑ 43.0 43.1 43.2 43.3 43.4 43.5 "Sex steroids and pulsatile luteinizing hormone release in men. Studies in estrogen-treated agonadal subjects and eugonadal subjects treated with a novel nonsteroidal antiandrogen". The Journal of Clinical Endocrinology and Metabolism 64 (4): 763–770. April 1987. doi:10.1210/jcem-64-4-763. PMID 3102546.
- ↑ 44.0 44.1 44.2 "Androgen action blockade does not result in reduction in size but changes histology of the normal human prostate". The Prostate 11 (4): 305–311. 1987. doi:10.1002/pros.2990110403. PMID 2960959.
- ↑ "The Influence of Hormone Treatment on Psychological Functioning of Transsexuals". Journal of Psychology & Human Sexuality 5 (4): 55–67. 1993. doi:10.1300/J056v05n04_04. ISSN 0890-7064.
- ↑ Drugs & Aging. Adis International. 1993. https://books.google.com/books?id=roFNAQAAIAAJ. "In 16 male subjects undergoing androgen blockade with nilutamide 100 to 300 mg/day for 8 weeks for male to female gender reassignment, prostate volume was not changed (de Voogt et al. 1987)."
- ↑ Translational Medicine: Molecular Pharmacology and Drug Discovery. Wiley. 2 March 2018. pp. 46–. ISBN 978-3-527-68719-0. https://books.google.com/books?id=kuhPDwAAQBAJ&pg=PA46.
- ↑ "Treatment of gynecomastia in patients with prostate cancer and androgen deprivation". Actas Urologicas Espanolas 38 (1): 34–40. 2014. doi:10.1016/j.acuroe.2013.10.002. PMID 23850393. "[...] the frequency of gynecomastia with antiandrogens in monotherapy is [...] around [...] 79% with nilutamide [...]".
- ↑ "Drug-induced gynecomastia: an evidence-based review". Expert Opinion on Drug Safety 11 (5): 779–795. September 2012. doi:10.1517/14740338.2012.712109. PMID 22862307. "Treatment with estrogen has the highest incidence of gynecomastia, at 40 – 80%, anti-androgens, including flutamide, bicalutamide and nilutamide, are next, with a 40 – 70% incidence, followed by GnRH analogs (goserelin, leuprorelin) and combined androgen deprivation [...]".
- ↑ "Images in clinical medicine. Gynecomastia induced by prostate-cancer treatment". The New England Journal of Medicine 367 (15): 1449. October 2012. doi:10.1056/NEJMicm1209166. PMID 23050528. "Gynecomastia occurs in up to 80% of patients who receive nonsteroidal antiandrogens (eg, bicalutamide, flutamide, or nilutamide), usually within the first 6 to 9 months after the initiation of treatment.".
- ↑ "Management of gynaecomastia in patients with prostate cancer: a systematic review". The Lancet. Oncology 6 (12): 972–979. December 2005. doi:10.1016/S1470-2045(05)70464-2. PMID 16321765.
- ↑ Drug-induced and Iatrogenic Respiratory Disease. CRC Press. 29 October 2010. pp. 235–. ISBN 978-1-4441-2869-7. https://books.google.com/books?id=QlJsBgAAQBAJ&pg=PA235.
- ↑ Contemporary Issues in Prostate Cancer: A Nursing Perspective. Jones & Bartlett Learning. 2006. pp. 257–. ISBN 978-0-7637-3075-8. https://books.google.com/books?id=dZe4ZSVDdBsC&pg=PA257.
- ↑ Prostate Cancer. Springer Science & Business Media. 5 June 2007. pp. 229–. ISBN 978-3-540-40901-4. https://books.google.com/books?id=Bg6ZbqhhboUC&pg=PA229.
- ↑ "A Review of the Clinical Studies with Nilutamide". Antiandrogens in Prostate Cancer. 1996. pp. 105–111. doi:10.1007/978-3-642-45745-6_10. ISBN 978-3-642-45747-0. "Akaza had to prematurely terminate a nilutamide study in Japan as 12.6% of his patients developed interstitial lung disease [4]. This complication has been mainly observed in Japan and much less in other trials worldwide."
- ↑ Micromedex (1 January 2003). USP DI 2003: Drug Information for Healthcare Professionals. Thomson Micromedex. pp. 220–224. ISBN 978-1-56363-429-1. https://books.google.com/books?id=zEzWtsVl-KgC.
- ↑ "Simultaneous liver and lung toxicity related to the nonsteroidal antiandrogen nilutamide (Anandron): a case report". The American Journal of Medicine 92 (5): 563–566. May 1992. doi:10.1016/0002-9343(92)90756-2. PMID 1580304.
- ↑ Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. 21 February 2009. pp. 150–. ISBN 978-0-08-093292-7. https://books.google.com/books?id=BWMeSwVwfTkC&pg=PA150.
- ↑ 59.0 59.1 "Tolerability of Nonsteroidal Antiandrogens in the Treatment of Advanced Prostate Cancer". The Oncologist 2 (1): 18–27. 1997. doi:10.1634/theoncologist.2-1-18. PMID 10388026. "Incidences of abnormal liver function test results have been variously reported from 2%-33% in nilutamide groups [13, 32, 33, 45] and from 4%-62% in flutamide groups [5, 7, 9, 11, 34, 38-40, 48] in trials of monotherapy and CAB.".
- ↑ Side Effects of Drugs Annual: A Worldwide Yearly Survey of New Data in Adverse Drug Reactions. Elsevier. 2011. pp. 874–. ISBN 978-0-444-53741-6. https://books.google.com/books?id=zegpAgEt3CcC&pg=PA874.
- ↑ "[Fatal fulminating hepatitis caused by nilutamide. A new case]" (in fr). Gastroenterologie Clinique et Biologique 20 (8–9): 710–711. 1996. PMID 8977826.
- ↑ 62.0 62.1 "Fulminant hepatic failure due to nilutamide hepatotoxicity". Digestive Diseases and Sciences 54 (4): 910–913. April 2009. doi:10.1007/s10620-008-0406-8. PMID 18688719. "In addition, nilutamide is noted to exhibit mitochondrial toxicity by inhibiting complex I activity of the mitochondrial respiratory chain leading to the impairment of ATP formation and the biosynthesis of glutathione, thereby possibly predisposing the liver to toxicity [13].".
- ↑ "Adverse Effects of Hormones and Hormone Antagonists on the Liver". Drug-Induced Liver Disease. 3. 2013. 605–619. doi:10.1016/B978-0-12-387817-5.00033-9. ISBN 9780123878175. "Liver injury is well recognized with all antiandrogens (Table 33-3). Thus, among all published cases identified between 1986 and 2003, flutamide (46), cyproterone (21), nilutamide (4), and bicalutamide (1) were implicated [107,108]."
- ↑ "Inhibition by nilutamide of the mitochondrial respiratory chain and ATP formation. Possible contribution to the adverse effects of this antiandrogen". The Journal of Pharmacology and Experimental Therapeutics 270 (1): 167–176. July 1994. PMID 8035313.
- ↑ 65.0 65.1 "Comparison of the cytotoxicity of the nitroaromatic drug flutamide to its cyano analogue in the hepatocyte cell line TAMH: evidence for complex I inhibition and mitochondrial dysfunction using toxicogenomic screening". Chemical Research in Toxicology 20 (9): 1277–1290. September 2007. doi:10.1021/tx7001349. PMID 17702527.
- ↑ "Bioactivation and hepatotoxicity of nitroaromatic drugs". Current Drug Metabolism 7 (7): 715–727. October 2006. doi:10.2174/138920006778520606. PMID 17073576.
- ↑ "Pharmacodynamics and Pharmacokinetics of Nilutamide in Animal and Man". Antiandrogens in Prostate Cancer. 1996. pp. 95–103. doi:10.1007/978-3-642-45745-6_9. ISBN 978-3-642-45747-0.
- ↑ 68.0 68.1 Drug Management of Prostate Cancer. Springer Science & Business Media. 14 September 2010. pp. 71–. ISBN 978-1-60327-829-4. https://books.google.com/books?id=4KDrjeWA5-UC&pg=PA71.
- ↑ Chronic Hyperandrogenic Anovulation. CRC Press. 15 December 1990. pp. 153–. ISBN 978-1-85070-322-8. https://books.google.com/books?id=q6zqFrCLUoIC&pg=PA153.
- ↑ "Design of antiandrogens and their mechanisms of action: a case study (anandron)". Hormone Research 28 (2–4): 230–241. 1987. doi:10.1159/000180948. PMID 3331376.
- ↑ "Action of a non-steroid anti-androgen, RU 23908, in peripheral and central tissues". Journal of Steroid Biochemistry 11 (1A): 93–99. July 1979. doi:10.1016/0022-4731(79)90281-4. PMID 385986.
- ↑ Clinical Manual of Psychopharmacology in the Medically Ill. American Psychiatric Pub. 20 May 2010. pp. 256–. ISBN 978-1-58562-942-8. https://books.google.com/books?id=9b5NkWZ5k8wC&pg=PA256.
- ↑ 73.0 73.1 "Nilutamide. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in prostate cancer". Drugs & Aging 3 (1): 9–25. 1993. doi:10.2165/00002512-199303010-00002. PMID 8453188.
- ↑ 74.0 74.1 "Inhibition of rat testicular 17 alpha-hydroxylase and 17,20-lyase activities by anti-androgens (flutamide, hydroxyflutamide, RU23908, cyproterone acetate) in vitro". Journal of Steroid Biochemistry 28 (1): 43–47. July 1987. doi:10.1016/0022-4731(87)90122-1. PMID 2956461.
- ↑ Antiandrogens in Prostate Cancer: A Key to Tailored Endocrine Treatment. Springer Science & Business Media. 6 December 2012. pp. 202–. ISBN 978-3-642-45745-6. https://books.google.com/books?id=jqZDBQAAQBAJ&pg=PT202. "The plateau level of nilutamide (steady state) was obtained after about 14 days of repeated administration of the drug (150 mg b.i.d.) and did not depend upon intervals between doses."
- ↑ "Clinical pharmacokinetics of the antiandrogens and their efficacy in prostate cancer". Clinical Pharmacokinetics 34 (5): 405–417. May 1998. doi:10.2165/00003088-199834050-00005. PMID 9592622.
- ↑ Successful Drug Discovery. Wiley. 16 April 2018. pp. 98–. ISBN 978-3-527-80868-7. https://books.google.com/books?id=t-JVDwAAQBAJ&pg=PA98.
- ↑ "Bicalutamide 150mg: a review of its use in the treatment of locally advanced prostate cancer". Drugs 66 (6): 837–850. 2006. doi:10.2165/00003495-200666060-00007. PMID 16706554.
- ↑ "Efficacy and advantages in the use of low doses of Anandron and estrogen combination in the treatment of prostate cancer". The Prostate 13 (1): 69–78. 1988. doi:10.1002/pros.2990130108. PMID 3420036.
- ↑ "Targeting the androgen receptor in breast cancer". Current Oncology Reports 17 (2): 4. February 2015. doi:10.1007/s11912-014-0427-8. PMID 25665553. http://handle.unsw.edu.au/1959.4/61991.
- ↑ "Phase II clinical and endocrine study of Anandron (RU-23908) in advanced post-menopausal breast cancer". British Journal of Cancer 63 (5): 763–764. May 1991. doi:10.1038/bjc.1991.170. PMID 1903951.
Further reading
- "The pure antiandrogen RU 23908 (Anandron), a candidate of choice for the combined antihormonal treatment of prostatic cancer: a review". The Prostate 5 (3): 299–311. 1984. doi:10.1002/pros.2990050307. PMID 6374639.
- "Pharmacological and clinical studies of the antiandrogen Anandron". Journal of Steroid Biochemistry 27 (4–6): 871–875. 1987. doi:10.1016/0022-4731(87)90162-2. PMID 3320565.
- "Castration plus nilutamide vs castration plus placebo in advanced prostate cancer. A review". Urology 37 (2 Suppl): 20–24. 1991. doi:10.1016/0090-4295(91)80097-q. PMID 1992599.
- "Pharmacokinetics and metabolism of nilutamide". Urology 37 (2 Suppl): 13–19. 1991. doi:10.1016/0090-4295(91)80096-p. PMID 1992598.
- "Nilutamide. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in prostate cancer". Drugs & Aging 3 (1): 9–25. 1993. doi:10.2165/00002512-199303010-00002. PMID 8453188.
- "Nilutamide: an antiandrogen for the treatment of prostate cancer". The Annals of Pharmacotherapy 31 (1): 65–75. January 1997. doi:10.1177/106002809703100112. PMID 8997470.
- "Nonsteroidal antiandrogens: a therapeutic option for patients with advanced prostate cancer who wish to retain sexual interest and function". BJU International 87 (1): 47–56. January 2001. doi:10.1046/j.1464-410x.2001.00988.x. PMID 11121992.
{{Navbox
| name = Androgens and antiandrogens | title = Androgens and antiandrogens | state = collapsed | listclass = hlist | groupstyle = text-align:center;
| group1 = Androgens
(incl. AAS)
| list1 =
| group2 = Antiandrogens | list2 = {{Navbox|child | groupstyle = text-align:center; | groupwidth = 9em;
| group1 = AR antagonists | list1 =
- Steroidal: Abiraterone acetate
- Canrenone
- Chlormadinone acetate
- Cyproterone acetate
- Delmadinone acetate
- Dienogest
- Drospirenone
- Medrogestone
- Megestrol acetate
- Nomegestrol acetate
- Osaterone acetate
- Oxendolone
- Potassium canrenoate
- Spironolactone
- Nonsteroidal: Apalutamide
- Bicalutamide
- Cimetidine
- Darolutamide
- Enzalutamide
- Flutamide
- Ketoconazole
- Nilutamide
- Seviteronel†
- Topilutamide (fluridil)
| group2 = Steroidogenesis| list2 =
inhibitors
| 5α-Reductase | |
|---|---|
| Others |
| group3 = Antigonadotropins | list3 =
- D2 receptor antagonists (prolactin releasers) (e.g., domperidone, metoclopramide, risperidone, haloperidol, chlorpromazine, sulpiride)
- Estrogens (e.g., bifluranol, [[diethylstilbestrol, estradiol, estradiol esters, ethinylestradiol, ethinylestradiol sulfonate, paroxypropione)
- GnRH agonists (e.g., leuprorelin)
- GnRH antagonists (e.g., cetrorelix)
- Progestogens (incl., chlormadinone acetate, [[cyproterone acetate, hydroxyprogesterone caproate, gestonorone caproate, [[Chemistry:Medroxyprogesterone medroxyprogesterone acetate, Chemistry:Megestrol acetate|megestrol acetate]])
| group4 = Others | list4 =
- Androstenedione immunogens: Androvax (androstenedione albumin)
- Ovandrotone albumin (Fecundin)
}}
| liststyle = background:#DDDDFF;| list3 =
- #WHO-EM
- ‡Withdrawn from market
- Clinical trials:
- †Phase III
- §Never to phase III
- See also
- Androgen receptor modulators
- Estrogens and antiestrogens
- Progestogens and antiprogestogens
- List of androgens/anabolic steroids
}}
 |

