Chemistry:Zopiclone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Imovane, Zimovane, Dopareel, others |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral tablets, 3.75 mg or 7.5mg (United Kingdom ), 5 mg, 7.5 mg, or 10 mg (Japan ) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 75–80%[1] |
| Protein binding | 52–59% |
| Metabolism | Hepatic through CYP3A4 and CYP2E1 |
| Elimination half-life | ~5 hours (3.5–6.5 hours) ~7–9 hours for 65+ years old |
| Excretion | Urine (80%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C17H17ClN6O3 |
| Molar mass | 388.81 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Zopiclone, sold under the brand name Imovane among others, is a nonbenzodiazepine used to treat difficulty sleeping. Zopiclone is molecularly distinct from benzodiazepine drugs and is classed as a cyclopyrrolone. However, zopiclone increases the normal transmission of the neurotransmitter gamma-aminobutyric acid (GABA) in the central nervous system, via modulating GABAA receptors similarly to the way benzodiazepine drugs do.
Zopiclone is a sedative. It works by causing a depression or tranquilization of the central nervous system. After prolonged use, the body can become accustomed to the effects of zopiclone. When the dose is then reduced or the drug is abruptly stopped, withdrawal symptoms may result. These can include a range of symptoms similar to those of benzodiazepine withdrawal. Although withdrawal symptoms from therapeutic doses of zopiclone and its isomers (i.e., eszopiclone) do not typically present with convulsions and are therefore not considered life-threatening, patients may experience such significant agitation or anxiety that they seek emergency medical attention. [citation needed]
In the United States, zopiclone is not commercially available,[2] although its active stereoisomer, eszopiclone, is. Zopiclone is a controlled substance in the United States, Japan, Brazil, New Zealand and some European countries, and may be illegal to possess without a prescription. [citation needed]
Zopiclone is known colloquially as a "Z-drug". Other Z-drugs include zaleplon and zolpidem and were initially thought to be less addictive than benzodiazepines. However, this appraisal has shifted somewhat in the last few years as cases of addiction and habituation have been presented. Zopiclone is recommended to be taken at the lowest effective dose, with a duration of 2–3 weeks for short-term insomnia.[3] Daily or continuous use of the drug is not usually advised, and caution must be taken when the compound is used in conjunction with antidepressants, sedatives or other drugs affecting the central nervous system.[4]
Medical uses

Zopiclone is used for the short-term treatment of insomnia where sleep initiation or sleep maintenance are prominent symptoms. Long-term use is not recommended, as tolerance, dependence, and addiction can occur.[5][6] One low-quality study found that zopiclone is ineffective in improving sleep quality or increasing sleep time in shift workers, and more research in this area has been recommended.[7]
Cognitive behavioral therapy has been found to be superior to zopiclone in the treatment of insomnia and has been found to have lasting effects on sleep quality for at least a year after therapy.[8][9][10][11]
Specific populations
Elderly
Zopiclone, similar to other benzodiazepines and nonbenzodiazepine hypnotic drugs, causes impairments in body balance and standing steadiness in individuals who wake up at night or the next morning. Falls and hip fractures are frequently reported. The combination with alcohol consumption increases these impairments. Partial, but incomplete tolerance develops to these impairments.[12] Zopiclone increases postural sway and increases the number of falls in older people, as well as cognitive side effects. Falls are a significant cause of death in older people.[13][14][15]
An extensive review of the medical literature regarding the management of insomnia and the elderly found that considerable evidence of the effectiveness and lasting benefits of nondrug treatments for insomnia exist. Compared with the benzodiazepines, the nonbenzodiazepine sedative-hypnotics, such as zopiclone, offer few if any advantages in efficacy or tolerability in elderly persons. Newer agents such as the melatonin receptor agonists may be more suitable and effective for the management of chronic insomnia in elderly people. Long-term use of sedative-hypnotics for insomnia lacks an evidence base and is discouraged for reasons that include concerns about such potential adverse drug effects as cognitive impairment (anterograde amnesia), daytime sedation, motor incoordination, and increased risk of motor vehicle accidents and falls. In addition, the effectiveness and safety of long-term use of nonbenzodiazepine hypnotic drugs remains to be determined.[16]
Liver disease
Patients with liver disease eliminate zopiclone much more slowly than normal patients and in addition experience exaggerated pharmacological effects of the drug.[17]
Adverse reactions
Sleeping pills, including zopiclone, have been associated with an increased risk of death.[18] The British National Formulary states adverse reactions as follows: "taste disturbance (some report a metallic taste); less commonly nausea, vomiting, dizziness, drowsiness, dry mouth, headache; rarely amnesia, confusion, depression, hallucinations, nightmares; very rarely light headedness, incoordination, paradoxical effects [...] and sleep-walking also reported".[19]
Contraindications
Zopiclone causes impaired driving skills similar to those of benzodiazepines. Long-term users of hypnotic drugs for sleep disorders develop only partial tolerance to adverse effects on driving, with users of hypnotic drugs even after one year of use still showing an increased motor vehicle accident rate.[20] Patients who drive motor vehicles should not take zopiclone as there is a significantly increased risk of accidents in zopiclone users.[21] Zopiclone induces impairment of psychomotor function.[22][23] Driving or operating machinery should be avoided after taking zopiclone as effects can carry over to the next day, including impaired hand-eye coordination.[24][25]
A double-blind study on the effect on performance of several hypnotic medications, relevant to military personnel who may have to be awakened to carry out duties, found that drugs listed in increasing order of performance impact duration were melatonin (with no impact), zaleplon, temazepam, and zopiclone. The effects on serial reaction time (SRT), logical reasoning (LRT), serial subtraction (SST), and multitask (MT) were measured. For zaleplon (10 mg), zopiclone (7.5 mg) and temazepam (15 mg) respectively the times to recover normal performance for SRT were 3.25, 6.25, and 5.25 hours; for LRT 3.25, >6.25, and 4.25 hours; for SST 2.25, >6.25, and 4.25 hours; and for MT 2.25, 4.25, and 3.25 hours. The study did not consider the effectiveness of the drugs on sleep.[26]
EEG and sleep
It causes similar alterations on EEG readings and sleep architecture as benzodiazepines and causes disturbances in sleep architecture on withdrawal as part of its rebound effect.[27][28] Zopiclone reduces both delta waves and the number of high-amplitude delta waves whilst increasing low-amplitude waves.[29] Zopiclone reduces the total amount of time spent in REM sleep as well as delaying its onset.[30][31] In EEG studies, zopiclone significantly increases the energy of the beta frequency band, increasing stage 2. Zopiclone is less selective to the α1 site and has higher affinity to the α2 site than zaleplon. Zopiclone is therefore very similar pharmacologically to benzodiazepines.[32]
Overdose
Zopiclone is sometimes used as a method of suicide.[33] It has a similar fatality index to that of benzodiazepine drugs, apart from temazepam, which is particularly toxic in overdose.[34][35] Deaths have occurred from zopiclone overdose, alone or in combination with other drugs.[36][37][38] Overdose of zopiclone may present with excessive sedation and depressed respiratory function that may progress to coma and possibly death.[39] Zopiclone combined with alcohol, opiates, or other central nervous system depressants may be even more likely to lead to fatal overdoses. Zopiclone overdosage can be treated with the GABAA receptor benzodiazepine site antagonist flumazenil, which displaces zopiclone from its binding site, thereby rapidly reversing its effects.[40][41] Serious effects on the heart may also occur from a zopiclone overdose[42][43] when combined with piperazine.[44]
Death certificates show the number of zopiclone-related deaths is on the rise.[45] When taken alone, it usually is not fatal, but when mixed with alcohol or other drugs such as opioids, or in patients with respiratory, or hepatic disorders, the risk of a serious and fatal overdose increases.[46][47]
Interactions
Zopiclone also interacts with trimipramine and caffeine.[48][49]
Alcohol has an additive effect when combined with zopiclone, enhancing the adverse effects including the overdose potential of zopiclone significantly.[50][51] Due to these risks and the increased risk for dependence, alcohol should be avoided when using zopiclone.[50]
Erythromycin appears to increase the absorption rate of zopiclone and prolong its elimination half-life, leading to increased plasma levels and more pronounced effects. Itraconazole has a similar effect on zopiclone pharmacokinetics as erythromycin. The elderly may be particularly sensitive to the erythromycin and itraconazole drug interaction with zopiclone. Temporary dosage reduction during combined therapy may be required, especially in the elderly.[52][53] Rifampicin causes a very notable reduction in half-life of zopiclone and peak plasma levels, which results in a large reduction in the hypnotic effect of zopiclone. Phenytoin and carbamazepine may also provoke similar interactions.[54] Ketoconazole and sulfaphenazole interfere with the metabolism of zopiclone.[55] Nefazodone impairs the metabolism of zopiclone leading to increased zopiclone levels and marked next-day sedation.[56]
Pharmacology
The therapeutic pharmacological properties of zopiclone include hypnotic, anxiolytic, anticonvulsant, and myorelaxant properties.[57] Zopiclone and benzodiazepines bind to the same sites on GABAA receptors, causing an enhancement of the actions of GABA to produce the therapeutic and adverse effects of zopiclone. The metabolite of zopiclone called desmethylzopiclone is also pharmacologically active, although it has predominately anxiolytic properties. One study found some slight selectivity for zopiclone on α1 and α5 subunits,[58] although it is regarded as being unselective in its binding to GABAA receptors containing α1, α2, α3, and α5 subunits. Desmethylzopiclone has been found to have partial agonist properties, unlike the parent drug zopiclone, which is a full agonist.[59] The mechanism of action of zopiclone is similar to benzodiazepines, with similar effects on locomotor activity and on dopamine and serotonin turnover.[60][61] A meta-analysis of randomised controlled clinical trials that compared benzodiazepines to zopiclone or other Z drugs such as zolpidem and zaleplon has found few clear and consistent differences between zopiclone and the benzodiazepines in sleep onset latency, total sleep duration, number of awakenings, quality of sleep, adverse events, tolerance, rebound insomnia, and daytime alertness.[62] Zopiclone is in the cyclopyrrolone family of drugs. Other cyclopyrrolone drugs include suriclone. Zopiclone, although molecularly different from benzodiazepines, shares an almost identical pharmacological profile as benzodiazepines, including anxiolytic properties. Its mechanism of action is by binding to the benzodiazepine site and acting as a full agonist, which in turn positively modulates benzodiazepine-sensitive GABAA receptors and enhances GABA binding at the GABAA receptors to produce zopiclone's pharmacological properties.[63][64][65] In addition to zopiclone's benzodiazepine pharmacological properties, it also has some barbiturate-like properties.[66][67]
Pharmacokinetics
After oral administration, zopiclone is rapidly absorbed, with a bioavailability around 75–80%. Time to peak plasma concentration is 1–2 hours. A high-fat meal preceding zopiclone administration does not change absorption (as measured by AUC), but reduces peak plasma levels and delays its occurrence, thus may delay the onset of therapeutic effects.
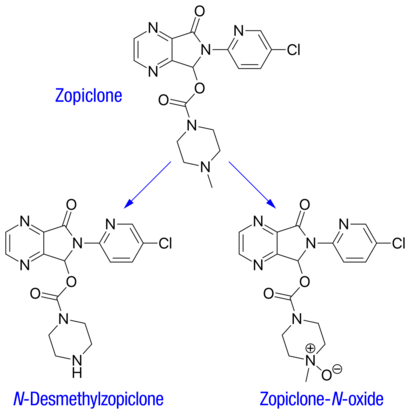
The plasma protein-binding of zopiclone has been reported to be weak, between 45 and 80% (mean 52–59%). It is rapidly and widely distributed to body tissues, including the brain, and is excreted in urine, saliva, and breast milk. Zopiclone is partly extensively metabolized in the liver to form an active N-demethylated derivative (N-desmethylzopiclone) and an inactive zopiclone-N-oxide. Hepatic enzymes playing the most significant role in zopiclone metabolism are CYP3A4 and CYP2E1. In addition, about 50% of the administered dose is decarboxylated and excreted via the lungs. In urine, the N-demethyl and N-oxide metabolites account for 30% of the initial dose. Between 7 and 10% of zopiclone is recovered from the urine, indicating extensive metabolism of the drug before excretion. The terminal elimination half-life of zopiclone ranges from 3.5 to 6.5 hours (5 hours on average).[1]
The pharmacokinetics of zopiclone in humans are stereoselective. After oral administration of the racemic mixture, Cmax (time to maximum plasma concentration), area under the plasma time-concentration curve (AUC) and terminal elimination half-life values are higher for the dextrorotatory enantiomers, owing to the slower total clearance and smaller volume of distribution (corrected by the bioavailability), compared with the levorotatory enantiomer. In urine, the concentrations of the dextrorotatory enantiomers of the N-demethyl and N-oxide metabolites are higher than those of the respective antipodes.
The pharmacokinetics of zopiclone are altered by aging and are influenced by renal and hepatic functions.[68] In severe chronic kidney failure, the area under the curve value for zopiclone was larger and the half-life associated with the elimination rate constant longer, but these changes were not considered to be clinically significant.[69] Sex and race have not been found to interact with pharmacokinetics of zopiclone.[1]
Chemistry
The melting point of zopiclone is 178 °C.[70] Zopiclone's solubility in water, at room temperature (25 °C) are 0.151 mg/mL.[70] The logP value of zopiclone is 0.8.[70]
Detection in biological fluids
Zopiclone may be measured in blood, plasma, or urine by chromatographic methods. Plasma concentrations are typically less than 100 μg/L during therapeutic use, but frequently exceed 100 μg/L in automotive vehicle operators arrested for impaired driving ability and may exceed 1000 μg/L in acutely poisoned patients. Post mortem blood concentrations are usually in a range of 400 to 3900 μg/L in victims of fatal acute overdose.[71][72][73]
History
Zopiclone was developed and first introduced in 1986 by Rhône-Poulenc S.A., now part of Sanofi, the main worldwide manufacturer. Initially, it was promoted as an improvement on benzodiazepines, but a recent meta-analysis found it was no better than benzodiazepines in any of the aspects assessed.[74] On April 4, 2005, the U.S. Drug Enforcement Administration listed zopiclone under schedule IV, due to evidence that the drug has addictive properties similar to benzodiazepines.
Zopiclone, as traditionally sold worldwide, is a racemic mixture of two stereoisomers, only one of which is active.[75][76] In 2005, the pharmaceutical company Sepracor of Marlborough, Massachusetts, began marketing the active stereoisomer eszopiclone under the name Lunesta in the United States. This had the consequence of placing what is a generic drug in most of the world under patent control in the United States. Generic forms of Lunesta have since become available in the United States. Zopiclone is currently available off-patent in a number of European countries, Brazil, Canada, Hong Kong, and New Zealand. The eszopiclone/zopiclone difference is in the dosage—the strongest eszopiclone dosage contains 3 mg of the therapeutic stereoisomer, whereas the highest zopiclone dosage (10 mg) contains 5 mg of the active stereoisomer[citation needed]. The two agents have not yet[when?] been studied in head-to-head clinical trials to determine the existence of any potential clinical differences (efficacy, side effects, developing dependence on the drug, safety, etc.).
Society and culture
Recreational use
Zopiclone has the potential for non-medical use, dosage escalation, and drug dependence. It is taken orally and sometimes intravenously when used non-medically, and often combined with alcohol to achieve euphoria. Patients abusing the drug are also at risk of dependence. Withdrawal symptoms can be seen after long-term use of normal doses even after a gradual reduction regimen. The Compendium of Pharmaceuticals and Specialties recommends zopiclone prescriptions not exceed 7 to 10 days, owing to concerns of addiction, tolerance, and physical dependence.[77] Two types of drug misuse can occur: either recreational misuse, wherein the drug is taken to achieve a high, or when the drug is continued long-term against medical advice.[78][79] Zopiclone may be more addictive than benzodiazepines.[80] Those with a history of substance misuse or mental health disorders may be at an increased risk of high-dose zopiclone misuse.[81] High dose misuse of zopiclone and increasing popularity amongst people who use substances who have been prescribed with zopiclone[82][clarification needed] The symptoms of zopiclone addiction can include depression, dysphoria, hopelessness, slow thoughts, social isolation, worrying, sexual anhedonia, and nervousness.[83]
Zopiclone and other sedative hypnotic drugs are detected frequently in cases of people suspected of driving under the influence of drugs. Other sedating drugs, including benzodiazepines and zolpidem, are also found in high numbers of suspected drugged drivers. Many drivers have blood levels far exceeding the therapeutic dose range and often in combination with alcohol, illegal, or addictive prescription drugs, suggesting a high degree of potential for non-medical use of benzodiazepines, zolpidem, and zopiclone.[84][85] Zopiclone, which at prescribed doses causes moderate impairment the next day, has been estimated to increase the risk of vehicle accidents by 50%, causing an increase of 503 excess accidents per 100,000 persons. Zaleplon or other nonimpairing sleep aids were recommended be used instead of zopiclone to reduce traffic accidents.[86] Zopiclone, as with other hypnotic drugs, is sometimes used to carry out criminal acts such as sexual assaults.[87]
Zopiclone has crosstolerance with barbiturates and is able to suppress barbiturate withdrawal symptoms. It is frequently self-administered intravenously in studies on monkeys, suggesting a high risk of addictive potential.[88]
Zopiclone is in the top ten medications obtained using a false prescription in France.[1]
References
- ↑ 1.0 1.1 1.2 1.3 "Assessment of Zopiclone". World Health Organization. 2006. p. 9 (Section 5. Pharmacokinetics). https://www.who.int/medicines/areas/quality_safety/4.6ZopicloneCritReview.pdf.
- ↑ "Zopiclone consumer information from". Drugs.com. https://www.drugs.com/cons/zopiclone.html.
- ↑ "Chapter 3 - The technologies, section 3.4", Clinical need and practice - Guidance on the use of zaleplon, zolpidem and zopiclone for the short-term management of insomnia, National Institute for Health and Care Excellence (NICE), 28 April 2004, Technology appraisal guidance [TA77], https://www.nice.org.uk/guidance/ta77/chapter/3-The-technologies, "This guidance will be reviewed if there is new evidence." Current as of 8 June 2023
- ↑ "Effects of zopiclone and temazepam on sleep, behaviour and mood during the day". European Journal of Clinical Pharmacology 36 (3): 247–251. 1989. doi:10.1007/BF00558155. ISSN 0031-6970. PMID 2744064.
- ↑ "What's wrong with prescribing hypnotics?". Drug and Therapeutics Bulletin 42 (12): 89–93. December 2004. doi:10.1136/dtb.2004.421289. PMID 15587763.
- ↑ "[Sleep disorders and hypnotic agents: medical, social and economical impact]" (in fr). Annales Pharmaceutiques Françaises 65 (4): 230–238. July 2007. doi:10.1016/s0003-4509(07)90041-3. PMID 17652991.
- ↑ "Pharmacological interventions for sleepiness and sleep disturbances caused by shift work". The Cochrane Database of Systematic Reviews 8 (8): CD009776. August 2014. doi:10.1002/14651858.CD009776.pub2. PMID 25113164.
- ↑ "Cognitive therapy superior to zopiclone for insomnia". The Journal of Family Practice 55 (10): 845. October 2006. PMID 17089469.
- ↑ "Discontinuation of benzodiazepines among older insomniac adults treated with cognitive-behavioural therapy combined with gradual tapering: a randomized trial". CMAJ 169 (10): 1015–1020. November 2003. PMID 14609970. PMC 236226. http://www.cmaj.ca/cgi/content/full/169/10/1015.
- ↑ "Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial". JAMA 295 (24): 2851–2858. June 2006. doi:10.1001/jama.295.24.2851. PMID 16804151.
- ↑ "Psychological treatment for insomnia in the regulation of long-term hypnotic drug use". Health Technology Assessment 8 (8): iii–iv, 1–68. February 2004. doi:10.3310/hta8080. PMID 14960254.
- ↑ "Effect of hypnotic drugs on body balance and standing steadiness". Sleep Medicine Reviews 14 (4): 259–267. August 2010. doi:10.1016/j.smrv.2009.10.008. PMID 20171127.
- ↑ "Effects of zopiclone, triazolam, and nitrazepam on standing steadiness". Neuropsychobiology 29 (1): 17–22. 1994. doi:10.1159/000119057. PMID 8127419.
- ↑ "Effects on postural oscillation and memory functions of a single dose of zolpidem 5 mg, zopiclone 3.75 mg and lormetazepam 1 mg in elderly healthy subjects. A randomized, cross-over, double-blind study versus placebo". European Journal of Clinical Pharmacology 59 (3): 179–188. July 2003. doi:10.1007/s00228-003-0591-5. PMID 12756510.
- ↑ "The art of prescribing. Risks and benefits of non-benzodiazepine receptor agonists in the treatment of acute primary insomnia in older adults". Perspectives in Psychiatric Care 42 (3): 196–200. August 2006. doi:10.1111/j.1744-6163.2006.00070.x. PMID 16916422.
- ↑ "Management of chronic insomnia in elderly persons". The American Journal of Geriatric Pharmacotherapy 4 (2): 168–192. June 2006. doi:10.1016/j.amjopharm.2006.06.006. PMID 16860264.
- ↑ "Plasma concentrations and central nervous system effects of the new hypnotic agent zopiclone in patients with chronic liver disease". British Journal of Clinical Pharmacology 16 (3): 259–265. September 1983. doi:10.1111/j.1365-2125.1983.tb02159.x. PMID 6626417.
- ↑ "Mortality Risk of Hypnotics: Strengths and Limits of Evidence". Drug Safety 39 (2): 93–107. February 2016. doi:10.1007/s40264-015-0362-0. PMID 26563222. https://escholarship.org/content/qt08d9f3d5/qt08d9f3d5.pdf?t=nz1gjv.
- ↑ "Zopiclone", British National Formulary (National Institute for Health and Care Excellence), 19 September 2016, https://www.evidence.nhs.uk/document?ci=https%3a%2f%2fwww.evidence.nhs.uk%2fformulary%2fbnf%2fcurrent%2f4-central-nervous-system%2f41-hypnotics-and-anxiolytics%2f411-hypnotics%2fzaleplon-zolpidem-and-zopiclone%2fzopiclone&returnUrl=Search%3fq%3dzopiclone&q=zopiclone, retrieved 2 October 2016
- ↑ "Next-day residual effects of hypnotics in DSM-IV primary insomnia: a driving simulator study with simultaneous electroencephalogram monitoring". Psychopharmacology 181 (4): 790–798. October 2005. doi:10.1007/s00213-005-0082-8. PMID 16025317.
- ↑ "Association of road-traffic accidents with benzodiazepine use". Lancet 352 (9137): 1331–1336. October 1998. doi:10.1016/S0140-6736(98)04087-2. PMID 9802269.
- ↑ "[Pharmacological profiles of benzodiazepinergic hypnotics and correlations with receptor subtypes]". Nihon Shinkei Seishin Yakurigaku Zasshi = Japanese Journal of Psychopharmacology 25 (3): 143–151. June 2005. PMID 16045197.
- ↑ "Effects of hypnotics on sleep and psychomotor performance. A double-blind randomised study of lormetazepam, midazolam and zopiclone". Anaesthesia 45 (12): 1079–1082. December 1990. doi:10.1111/j.1365-2044.1990.tb14896.x. PMID 2278337.
- ↑ "A double-blind study to establish the residual effects of zopiclone on performance in healthy volunteers". International Pharmacopsychiatry 17 (Suppl 2): 98–108. 1982. PMID 7188379.
- ↑ "Dose-response effects of zopiclone on night sleep and on nighttime and daytime functioning". Sleep 10 (Suppl 1): 27–34. 1987. doi:10.1093/sleep/10.suppl_1.27. PMID 3326113.
- ↑ "Impact of Melatonin, Zaleplon, Zopiclone, and Temazepam on Psychomotor Performance". Aviation, Space, and Environmental Medicine (Aerospace Medical Association) 74 (12): 1263-1270. December 2003. https://www.researchgate.net/publication/8945690_Impact_of_Melatonin_Zaleplon_Zopiclone_and_Temazepam_on_Psychomotor_Performance.
- ↑ "Effect of zopiclone and midazolam on sleep and EEG spectra in a phase-advanced sleep schedule". Neuropsychopharmacology 3 (1): 11–18. February 1990. PMID 2306331.
- ↑ "Acute, subchronic and discontinuation effects of zopiclone on sleep EEG and nocturnal melatonin secretion". Eur Neuropsychopharmacol 6 (3): 163–168. August 1996. doi:10.1016/0924-977X(96)00014-4. PMID 8880074.
- ↑ "Modulation of delta activity by hypnotics in middle-aged subjects: studies with a benzodiazepine (flurazepam) and a cyclopyrrolone (zopiclone)". Sleep 9 (2): 348–352. June 1986. doi:10.1093/sleep/9.2.348. PMID 3505734.
- ↑ "Comparison of the effect of zopiclone and brotizolam on sleep EEG by quantitative evaluation in healthy young women". Sleep 16 (7): 655–661. October 1993. doi:10.1093/sleep/16.7.655. PMID 8290860.
- ↑ "Effects of zopiclone, flunitrazepam, triazolam and levomepromazine on the transient change in sleep-wake schedule: polygraphic study, and the evaluation of sleep and daytime condition". Prog. Neuropsychopharmacol. Biol. Psychiatry 17 (2): 229–239. March 1993. doi:10.1016/0278-5846(93)90044-S. PMID 8430216.
- ↑ "Electroencephalographic properties of zaleplon, a non-benzodiazepine sedative/hypnotic, in rats". Journal of Pharmacological Sciences 94 (3): 246–251. March 2004. doi:10.1254/jphs.94.246. PMID 15037809.
- ↑ "Detection and quantification of the hypnotic zopiclone, connected with an uncommon case of drowning". Forensic Science International 83 (1): 67–72. November 1996. doi:10.1016/0379-0738(96)02018-X. PMID 8939015.
- ↑ Buckley NA, Dawson AH, Whyte IM, McManus P, Ferguson N.Correlations between prescriptions and drugs taken in self-poisoning: Implications for prescribers and drug regulation.Med J Aust (in press)
- ↑ "Relative toxicity of benzodiazepines in overdose". BMJ 310 (6974): 219–221. January 1995. doi:10.1136/bmj.310.6974.219. PMID 7866122.
- ↑ "Zopiclone fatality in a hospitalized patient". Journal of Forensic Sciences 42 (2): 340–343. March 1997. doi:10.1520/JFS14125J. PMID 9068198.
- ↑ "Analysis of zopiclone (Imovane) in postmortem specimens by GC-MS and HPLC with diode-array detection". Journal of Analytical Toxicology 20 (1): 52–54. 1996. doi:10.1093/jat/20.1.52. PMID 8837952.
- ↑ "[An autopsy case of poisoning by neuropsychopharmaceuticals including zopiclone]" (in ja). Nihon Hoigaku Zasshi = the Japanese Journal of Legal Medicine 52 (4): 245–252. August 1998. PMID 9893443.
- ↑ "Two cases of fatal zopiclone overdose". Journal of Analytical Toxicology 20 (2): 131–133. 1996. doi:10.1093/jat/20.2.131. PMID 8868406.
- ↑ "Zopiclone overdose responsive to flumazenil". Clinical Toxicology 43 (5): 385–386. 2005. doi:10.1081/clt-200058944. PMID 16235515.
- ↑ "Zopiclone poisoning: tissue distribution and potential for postmortem diffusion". Forensic Science International 65 (3): 177–183. May 1994. doi:10.1016/0379-0738(94)90273-9. PMID 8039775.
- ↑ "[First-degree heart block caused by voluntary zopiclone poisoning]" (in fr). Therapie 45 (2): 162. 1990. PMID 2353332.
- ↑ "[Auriculo-ventricular block during voluntary poisoning with zopiclone]" (in fr). Therapie 44 (5): 379–380. 1989. PMID 2814922.
- ↑ Medical Toxicology. 2003. p. 889. ISBN 978-0-7817-2845-4.
- ↑ "The role of benzodiazepines in elderly suicides". Scandinavian Journal of Public Health 31 (3): 224–228. 2003. doi:10.1080/14034940210167966. PMID 12850977.
- ↑ "[Acute poisoning by new psychotropic drugs]". La Revue du Praticien 47 (7): 731–735. April 1997. PMID 9183949.
- ↑ "Fatal overdose of zopiclone in an elderly woman with bronchogenic carcinoma". Journal of Forensic Sciences 46 (5): 1247–1249. September 2001. doi:10.1520/JFS15131J. PMID 11569575.
- ↑ "Pharmacokinetic and clinical parameters of zopiclone and trimipramine when administered simultaneously to volunteers". Biopharmaceutics & Drug Disposition 5 (2): 117–125. April 1984. doi:10.1002/bdd.2510050205. PMID 6743780.
- ↑ "Caffeine moderately antagonizes the effects of triazolam and zopiclone on the psychomotor performance of healthy subjects". Pharmacology & Toxicology 70 (4): 286–289. April 1992. doi:10.1111/j.1600-0773.1992.tb00473.x. PMID 1351673.
- ↑ 50.0 50.1 "Actions and interactions of hypnotics on human performance: single doses of zopiclone, triazolam and alcohol". International Clinical Psychopharmacology 5 (Suppl 2): 115–130. April 1990. PMID 2201724.
- ↑ "Interaction of alcohol and drugs in fatal poisonings". Human & Experimental Toxicology 22 (5): 281–287. May 2003. doi:10.1191/0960327103ht324oa. PMID 12774892.
- ↑ "The effect of erythromycin on the pharmacokinetics and pharmacodynamics of zopiclone". British Journal of Clinical Pharmacology 38 (4): 363–367. October 1994. doi:10.1111/j.1365-2125.1994.tb04367.x. PMID 7833227.
- ↑ "Effect of itraconazole on the pharmacokinetics and pharmacodynamics of zopiclone". European Journal of Clinical Pharmacology 51 (3–4): 331–334. 1996. doi:10.1007/s002280050207. PMID 9010708.
- ↑ "Concentrations and effects of zopiclone are greatly reduced by rifampicin". British Journal of Clinical Pharmacology 43 (5): 471–474. May 1997. doi:10.1046/j.1365-2125.1997.00579.x. PMID 9159561.
- ↑ "Cytochrome P-450 3A4 and 2C8 are involved in zopiclone metabolism". Drug Metabolism and Disposition 27 (9): 1068–1073. September 1999. PMID 10460808. http://dmd.aspetjournals.org/cgi/content/full/27/9/1068. Retrieved 2008-12-16.
- ↑ "Possible interaction of zopiclone and nefazodone". The Annals of Pharmacotherapy 35 (11): 1378–1380. November 2001. doi:10.1345/aph.1A074. PMID 11724087.[yes|permanent dead link|dead link}}]
- ↑ "Functional properties of the brain during sleep under subchronic zopiclone administration in man". European Neuropsychopharmacology 4 (1): 21–30. March 1994. doi:10.1016/0924-977X(94)90311-5. PMID 8204993.
- ↑ "Indiplon is a high-affinity positive allosteric modulator with selectivity for alpha1 subunit-containing GABAA receptors". The Journal of Pharmacology and Experimental Therapeutics 317 (1): 369–377. April 2006. doi:10.1124/jpet.105.096701. PMID 16399882.
- ↑ "Anxioselective compounds acting at the GABA(A) receptor benzodiazepine binding site". Current Drug Targets. CNS and Neurological Disorders 2 (4): 213–232. August 2003. doi:10.2174/1568007033482841. PMID 12871032.
- ↑ "Pharmacologic studies of the central action of zopiclone: effects on locomotor activity and brain monoamines in rats". International Journal of Clinical Pharmacology, Therapy, and Toxicology 23 (3): 121–128. March 1985. PMID 2581904.
- ↑ "Pharmacologic studies of central actions of zopiclone: influence on brain monoamines in rats under stressful condition". International Journal of Clinical Pharmacology, Therapy, and Toxicology 23 (4): 204–210. April 1985. PMID 2860074.
- ↑ "Comparative efficacy of newer hypnotic drugs for the short-term management of insomnia: a systematic review and meta-analysis". Human Psychopharmacology 19 (5): 305–322. July 2004. doi:10.1002/hup.594. PMID 15252823.
- ↑ "Suriclone: a new cyclopyrrolone derivative recognizing receptors labeled by benzodiazepines in rat hippocampus and cerebellum". Journal of Neurochemistry 40 (3): 601–607. March 1983. doi:10.1111/j.1471-4159.1983.tb08023.x. PMID 6298365.
- ↑ "Enhancement of GABA binding by benzodiazepines and related anxiolytics". European Journal of Pharmacology 89 (3–4): 193–198. May 1983. doi:10.1016/0014-2999(83)90494-6. PMID 6135616.
- ↑ "Effects of non-sedative anxiolytic drugs on responses to GABA and on diazepam-induced enhancement of these responses on mouse neurones in cell culture". British Journal of Pharmacology 95 (1): 109–120. September 1988. doi:10.1111/j.1476-5381.1988.tb16554.x. PMID 2905900.
- ↑ "Pharmacological studies on zopiclone". Pharmacology 27 (Suppl 2): 46–58. 1983. doi:10.1159/000137911. PMID 6142468.
- ↑ "Brain receptors and zopiclone". Pharmacology 27 (Suppl 2): 59–69. 1983. doi:10.1159/000137912. PMID 6322210.
- ↑ "Pharmacokinetics and metabolism of zopiclone". International Pharmacopsychiatry 17 (Suppl 2): 76–91. 1982. PMID 7188377.
- ↑ "Steady state pharmacokinetics of zopiclone during multiple oral dosing (7.5 mg nocte) in patients with severe chronic renal failure". International Clinical Psychopharmacology 5 (Suppl 2): 95–104. April 1990. PMID 2387982.
- ↑ 70.0 70.1 70.2 "Zopiclone" (in en). U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/5735.
- ↑ "Screening, library-assisted identification and validated quantification of 23 benzodiazepines, flumazenil, zaleplone, zolpidem and zopiclone in plasma by liquid chromatography/mass spectrometry with atmospheric pressure chemical ionization". Journal of Mass Spectrometry 39 (8): 856–72. August 2004. doi:10.1002/jms.599. PMID 15329838. Bibcode: 2004JMSp...39..856K.
- ↑ "Impairment related to blood drug concentrations of zopiclone and zolpidem compared to alcohol in apprehended drivers". Accident Analysis and Prevention 41 (3): 462–6. May 2009. doi:10.1016/j.aap.2009.01.011. PMID 19393793.
- ↑ Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. 2008. pp. 1677–1679.
- ↑ "Meta-analysis of benzodiazepine use in the treatment of insomnia". CMAJ 162 (2): 225–233. January 2000. PMID 10674059.
- ↑ "Preparative and analytical separation of the zopiclone enantiomers and determination of their affinity to the benzodiazepine receptor binding site". Chirality 5 (6): 419–421. 1993. doi:10.1002/chir.530050605. PMID 8398600.
- ↑ "Pharmacokinetics of zopiclone and its enantiomers in Caucasian young healthy volunteers". Drug Metabolism and Disposition 21 (6): 1125–1128. November 1993. PMID 7905394.
- ↑ "Zopiclone: is it a pharmacologic agent for abuse?". Canadian Family Physician 53 (12): 2124–2129. December 2007. PMID 18077750.
- ↑ "Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds". The Journal of Clinical Psychiatry 66 (Suppl 9): 31–41. 2005. PMID 16336040.
- ↑ "[High usage of zolpidem and zopiclone. Cross-sectional study using claims data]" (in de). Der Nervenarzt 79 (1): 67–72. January 2008. doi:10.1007/s00115-007-2280-6. PMID 17457554.
- ↑ "[Adverse effects of zopiclone]" (in no). Tidsskrift for den Norske Laegeforening 118 (13): 2029–2032. May 1998. PMID 9656789.
- ↑ "[Dependency of non-benzodiazepine hypnotics. Two case reports]" (in de). Der Nervenarzt 70 (1): 72–75. January 1999. doi:10.1007/s001150050403. PMID 10087521.
- ↑ "Physical dependence on zopiclone. Prescribing this drug to addicts may give rise to iatrogenic drug misuse". BMJ 317 (7151): 146. July 1998. doi:10.1136/bmj.317.7151.146. PMID 9657802.
- ↑ "Excessive use of zopiclone: a case report". Swiss Medical Weekly 132 (35–36): 523. September 2002. doi:10.4414/smw.2002.10074. PMID 12506335. http://www.smw.ch/docs/pdf200x/2002/35/smw-10074.pdf. Retrieved 2008-12-18.
- ↑ "Concentrations of scheduled prescription drugs in blood of impaired drivers: considerations for interpreting the results". Therapeutic Drug Monitoring 29 (2): 248–260. April 2007. doi:10.1097/FTD.0b013e31803d3c04. PMID 17417081.
- ↑ "[Detection of zopiclone in many drivers--a sign of misuse or abuse]" (in no). Tidsskrift for den Norske Laegeforening 119 (19): 2820–2821. August 1999. PMID 10494203.
- ↑ "A general model of the effects of sleep medications on the risk and cost of motor vehicle accidents and its application to France". PharmacoEconomics 19 (1): 69–78. January 2001. doi:10.2165/00019053-200119010-00005. PMID 11252547.
- ↑ "Testing for the undetectable in drug-facilitated sexual assault using hair analyzed by tandem mass spectrometry as evidence". Therapeutic Drug Monitoring 26 (2): 211–214. April 2004. doi:10.1097/00007691-200404000-00022. PMID 15228167.
- ↑ "Dependence potential of zopiclone studied in monkeys". International Pharmacopsychiatry 17 (2): 216–227. 1982. PMID 6892368.
External links
- Detailed pharmacological information
- Scheduling recommendation (PDF file)
- Details on scheduling
- Erowid zopiclone vault
- Support for zopiclone dependency/addiction
 |
