Chemistry:Gamma-Valerolactone
| |
| |
| Names | |
|---|---|
| IUPAC name
5-Methyloxolan-2-one
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 80420 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 1224 |
| |
| |
| Properties | |
| C5H8O2 | |
| Molar mass | 100.116 |
| Appearance | colorless liquid |
| Density | 1.0546 g/mL (20 °C) [2] |
| Melting point | −31 °C (−24 °F; 242 K) |
| Boiling point | 205 °C (401 °F; 478 K)[2] |
| >=100 mg/mL | |
Refractive index (nD)
|
1.4333 (20 °C) [2] |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-461.3 kJ·mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
-2649.6 kJ·mol−1 |
| Hazards[3] | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H319 | |
| NFPA 704 (fire diamond) | |
| Flash point | 81 °C (178 °F; 354 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
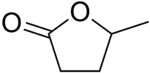
γ-Valerolactone (GVL) is an organic compound with the formula C5H8O2. This colourless liquid is one of the more common lactones. GVL is chiral but is usually used as the racemate. It is readily obtained from cellulosic biomass and is a potential fuel and green solvent.
GVL behaves as a prodrug to γ-hydroxyvaleric acid (GHV), a drug with similar effects to those of γ-hydroxybutyric acid (GHB), albeit with less potency in comparison.[4] Because GHB is controlled in many parts of the world, while GVL is not, GVL has gained popularity as a legal substitute for GHB.[4][5]
Synthesis
GVL is produced from levulinic acid, which is obtained from hexoses. In a typical process, cellulosic biomasses, such as corn stover, sawgrass, or wood, is hydrolysed into glucose and other sugars using acid catalysts. The resulting glucose can then be dehydrated via hydroxymethylfurfural to yield formic acid and levulinic acid, which cyclises to intermediate unsaturated ring compounds, which can then be hydrogenated to gamma-valerolactone, which has potential applications as a liquid fuel.[6]
Potential applications
GVL has been identified as a potential green solvent. Because of its herbal odor, it is used in the perfume and flavor industries.[7] It is a structural isomer of δ-valerolactone.
Potential fuel
Since it is readily obtained from glucose, GVL has long been identified as a potential "green fuel."[8] GVL retains 97% of the energy of glucose and can be blended by itself in gasoline where it performs comparably to ethanol/gasoline mixtures.[9][10] However, due to blending limits for use in conventional combustion engines, it may be more efficient to convert GVL into liquid alkenes (or alkanes). The first step in this process is the ring-opening of GVL to yield a mixture of pentenoic acids. These acids can then be decarboxylated to produce butene and CO2. These conversions can be performed with zeolite catalysts.[11] After this stream is dehydrated, the products can be oligomerized at elevated pressures in the presence of a common acid-catalyst to yield alkenes with higher molecular weights, targeted for gasoline and other fuel applications.[12]
One of the main advantages that allows GVL to be a practical biofuel is that it is relatively inexpensive to produce. Using a cheap feedstock, this biofuel can be produced at prices between 2-3 US$/gallon.[9] The conversion of GVL to transportation fuel capable alkenes only requires a system containing two flow reactors, two phase separators, and a simple pumping arrangement for the delivery of an aqueous GVL feed. Since the use of precious metal catalysts is not required, this also decreases the total price of fuel production.[11]
Potential production of biomass-derived fuels
Apart from its value as a potential fuel in its own right, gamma-valerolactone has shown promise in laboratory-scale thermocatalytic production of soluble carbohydrates from corn stover and wood at high yields. The biomass reacts in a solvent mixture of water, dilute sulfuric acid, and gamma-valerolactone, itself derived from biomass. The gamma-valerolactone promotes thermocatalytic hydrolysis into monosaccharides by complete solubilization of the raw material, including lignins. The saccharide products can be recovered from the lactone into water solution by antisolvent addition of salt or liquid carbon dioxide. The product can be used as feedstock for producing furans or ethanol at high yield, while the gamma-valerolactone is returned to the catalytic cycle.[13]
Membrane Fabrication
Gamma-Valerolactone has been studied and shown the potential to prepare dope solutions for the fabrication of polymeric membranes. Due to the toxicity of the traditional solvents, green solvents were investigated in recent years.[14] Due to its environmentally friendly profile, Gamma-Valerolactone showed the potential to fabricate polysulfone membranes as a co-solvent.[15]
See also
- δ-Valerolactone
- Valeric acid
- 1,4-Butanediol (1,4-BD)
- γ-Butyrolactone (GBL)
References
- ↑ NIH National Toxicology Program
- ↑ 2.0 2.1 2.2 Baird, Zachariah Steven; Uusi-Kyyny, Petri; Pokki, Juha-Pekka; Pedegert, Emilie; Alopaeus, Ville (6 Nov 2019). "Vapor Pressures, Densities, and PC-SAFT Parameters for 11 Bio-compounds". International Journal of Thermophysics 40 (11): 102. doi:10.1007/s10765-019-2570-9. Bibcode: 2019IJT....40..102B.
- ↑ "Summary of Classification and Labelling". https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/45940.
- ↑ 4.0 4.1 "Uptake of gamma-valerolactone--detection of gamma-hydroxyvaleric acid in human urine samples". J Anal Toxicol 37 (4): 250–4. 2013. doi:10.1093/jat/bkt013. PMID 23486087.
- ↑ Fred Smith (31 December 2004). Handbook of Forensic Drug Analysis. Academic Press. pp. 462–. ISBN 978-0-08-047289-8. https://books.google.com/books?id=8JsQgRO3QcwC&pg=PA462.
- ↑ Huber, George W.; Iborra, Sara; Corma, Avelino (2006). "Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering". Chemical Reviews 106 (9): 4044–4098. doi:10.1021/cr068360d. PMID 16967928. https://works.bepress.com/cgi/viewcontent.cgi?article=1058&context=george_huber.
- ↑ GoodScentsCompany.com
- ↑ Huber, G. W.; Corma, Avelino (2007). "Synergies between Bio- and Oil Refineries for the Production of Fuels from Biomass". Angewandte Chemie International Edition 46 (38): 7184–7201. doi:10.1002/anie.200604504. PMID 17610226. https://works.bepress.com/cgi/viewcontent.cgi?article=1054&context=george_huber.
- ↑ 9.0 9.1 Savage, Neil (2011). "Fuel Options: The Ideal Biofuel". Nature 474 (7352): S9–S11. doi:10.1038/474S09a. PMID 21697843.
- ↑ Horváth, I. T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L. T. (2008). "γ-Valerolactone—a sustainable liquid for energy and carbon-based chemicals". Green Chemistry 10 (2): 238–242. doi:10.1039/b712863k.
- ↑ 11.0 11.1 Bond, Jesse Q.; Alonso, David Martin; Wang, Dong; West, Ryan M.; Dumesic, James A. (2010). "Integrated Catalytic Conversion of γ-Valerolactone to Liquid Alkenes for Transportation Fuels". Science 327 (5969): 1110–1114. doi:10.1126/science.1184362. PMID 20185721. Bibcode: 2010Sci...327.1110B.
- ↑ Mantilla, A. et al. (2005). "Oligomerization of isobutene on sulfated titania: Effect of reaction conditions on selectivity". Catalysis Today 107–108: 707–712. doi:10.1016/j.cattod.2005.07.153.
- ↑ Luterbacher, Jeremy S.Expression error: Unrecognized word "etal". (2014). "Nonenzymatic Sugar Production from Biomass Using Biomass-Derived gamma-Valerolactone". Science 343 (6168): 277–280. doi:10.1126/science.1246748. PMID 24436415. Bibcode: 2014Sci...343..277L.
- ↑ Figoli, A.; Marino, T.; Simone, S.; Di Nicolò, E.; Li, X.-M.; He, T.; Tornaghi, S.; Drioli, E. (2014-07-03). "Towards non-toxic solvents for membrane preparation: a review" (in en). Green Chemistry 16 (9): 4034. doi:10.1039/c4gc00613e. ISSN 1463-9262.
- ↑ Dong, Xiaobo; Shannon, Halle D.; Escobar, Isabel C. (January 2018), "Investigation of PolarClean and Gamma-Valerolactone as Solvents for Polysulfone Membrane Fabrication" (in EN), Green Polymer Chemistry: New Products, Processes, and Applications (American Chemical Society): pp. 385–403, doi:10.1021/bk-2018-1310.ch024, ISBN 978-0841233898
External links
- General Safety Information
{{Navbox | name = GABA receptor modulators | title = GABA receptor modulators | state = collapsed | bodyclass = hlist | groupstyle = text-align:center;
| group1 = Ionotropic | list1 = {{Navbox|subgroup | groupstyle = text-align:center | groupwidth = 5em
| group1 = GABAA | list1 =
- Agonists: (+)-Catechin
- Bamaluzole
- Barbiturates (e.g., phenobarbital)
- BL-1020
- DAVA
- Dihydromuscimol
- GABA
- Gabamide
- GABOB
- Gaboxadol (THIP)
- Homotaurine (tramiprosate, 3-APS)
- Ibotenic acid
- iso-THAZ
- iso-THIP
- Isoguvacine
- Isomuscimol
- Isonipecotic acid
- Kojic amine
- Lignans (e.g., honokiol)
- Methylglyoxal
- Monastrol
- Muscimol
- Nefiracetam
- Neuroactive steroids (e.g., allopregnanolone)
- Org 20599
- PF-6372865
- Phenibut
- Picamilon
- P4S
- Progabide
- Propofol
- Quisqualamine
- SL-75102
- TACA
- TAMP
- Terpenoids (e.g., borneol)
- Thiomuscimol
- Tolgabide
- ZAPA
- Positive modulators (abridged; see here for a full list): α-EMTBL
- Alcohols (e.g., ethanol)
- Anabolic steroids
- Avermectins (e.g., ivermectin)
- Barbiturates (e.g., phenobarbital)
- Benzodiazepines (e.g., diazepam)
- Bromide compounds (e.g., potassium bromide)
- Carbamates (e.g., meprobamate)
- Carbamazepine
- Chloralose
- Chlormezanone
- Clomethiazole
- Dihydroergolines (e.g., ergoloid (dihydroergotoxine))
- Etazepine
- Etifoxine
- Fenamates (e.g., mefenamic acid)
- Flavonoids (e.g., apigenin, hispidulin)
- Fluoxetine
- Flupirtine
- Imidazoles (e.g., etomidate)
- Kava constituents (e.g., kavain)<!--PMID: 9776662-->
- Lanthanum
- Loreclezole
- Monastrol
- Neuroactive steroids (e.g., allopregnanolone, [[Chemistry:Cholecholesterol]], THDOC)
- Niacin
- Nicotinamide (niacinamide)
- Nonbenzodiazepines (e.g., β-carbolines (e.g., [[abecarnil), cyclopyrrolones (e.g., zopiclone), imidazopyridines (e.g., zolpidem), pyrazolopyrimidines (e.g., zaleplon))
- Norfluoxetine
- Petrichloral
- Phenols (e.g., propofol)
- Phenytoin
- Piperidinediones (e.g., glutethimide)
- Propanidid
- Pyrazolopyridines (e.g., etazolate)
- Quinazolinones (e.g., methaqualone)
- Retigabine (ezogabine)
- ROD-188
- Skullcap constituents (e.g., baicalin)
- Stiripentol
- Sulfonylalkanes (e.g., sulfonmethane (sulfonal))
- Topiramate
- Valerian constituents (e.g., valerenic acid)
- Volatiles/gases (e.g., chloral hydrate, chloroform, [[Chemistry:Diethyl diethyl ether, Parparaldehyde]], sevoflurane)
- Antagonists: Bicuculline
- Coriamyrtin
- Dihydrosecurinine
- Gabazine (SR-95531)
- Hydrastine
- Hyenachin (mellitoxin)
- PHP-501
- Pitrazepin
- Securinine
- Sinomenine
- SR-42641
- SR-95103
- Thiocolchicoside
- Tutin
- Negative modulators: 1,3M1B
- 3M2B
- 11-Ketoprogesterone
- 17-Phenylandrostenol
- α5IA (LS-193,268)
- β-CCB
- β-CCE
- β-CCM
- β-CCP
- β-EMGBL
- Anabolic steroids
- Amiloride
- Anisatin
- β-Lactams (e.g., penicillins, cephalosporins, carbapenems)
- Basmisanil
- Bemegride
- Bicyclic phosphates (TBPS, TBPO, IPTBO)
- BIDN
- Bilobalide
- Bupropion
- CHEB
- Chlorophenylsilatrane
- Cicutoxin
- Cloflubicyne
- Cyclothiazide
- DHEA
- DHEA-S
- Dieldrin
- (+)-DMBB
- DMCM
- DMPC
- EBOB
- Etbicyphat
- FG-7142 (ZK-31906)
- Fiproles (e.g., fipronil)
- Flavonoids (e.g., amentoflavone, oroxylin A)
- Flumazenil
- Fluoroquinolones (e.g., ciprofloxacin)
- Flurothyl
- Furosemide
- Golexanolone
- Iomazenil (123I)
- IPTBO
- Isopregnanolone (sepranolone)
- L-655,708
- Laudanosine
- Leptazol
- Lindane
- MaxiPost
- Morphine
- Morphine-3-glucuronide
- MRK-016
- Naloxone
- Naltrexone
- Nicardipine
- Nonsteroidal antiandrogens (e.g., [[apalutamide, [[Chemistry:Bicalutbicalutamide, Enzalutenzalutamide, Chemistry:Flutamide|flut]]amide]], nilutamide)
- Oenanthotoxin
- Pentylenetetrazol (pentetrazol)
- Phenylsilatrane
- Picrotoxin (i.e., picrotin, picrotoxinin and dihydropicrotoxinin)
- Pregnenolone sulfate
- Propybicyphat
- PWZ-029
- Radequinil
- Ro 15-4513
- Ro 19-4603
- RO4882224
- RO4938581
- Sarmazenil
- SCS
- Suritozole
- TB-21007
- TBOB
- TBPS
- TCS-1105
- Terbequinil
- TETS
- Thujone
- U-93631
- Zinc
- ZK-93426
| group2 = GABAA-ρ | list2 =
- Agonists: BL-1020
- CACA
- CAMP
- Homohypotaurine
- GABA
- GABOB
- Ibotenic acid
- Isoguvacine
- Muscimol
- N4-Chloroacetylcytosine arabinoside
- Picamilon
- Progabide
- TACA
- TAMP
- Thiomuscimol
- Tolgabide
- Positive modulators: Allopregnanolone
- Alphaxolone
- ATHDOC
- Lanthanides
- Antagonists: (S)-2-MeGABA
- (S)-4-ACPBPA
- (S)-4-ACPCA
- 2-MeTACA
- 3-APMPA
- 4-ACPAM
- 4-GBA
- cis-3-ACPBPA
- CGP-36742 (SGS-742)
- DAVA
- Gabazine (SR-95531)
- Gaboxadol (THIP)
- I4AA
- Isonipecotic acid
- Loreclezole
- P4MPA
- P4S
- SKF-97541
- SR-95318
- SR-95813
- TPMPA
- trans-3-ACPBPA
- ZAPA
- Negative modulators: 5α-Dihydroprogesterone
- Bilobalide
- Loreclezole
- Picrotoxin (picrotin, picrotoxinin)
- Pregnanolone
- ROD-188
- THDOC
- Zinc
}}
| group2 = Metabotropic
| list2 =



