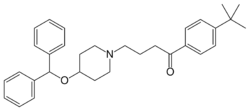
Chemistry:Ebastine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | Greater than 95% |
| Metabolism | Hepatic (CYP3A4-mediated), to carebastine |
| Elimination half-life | 15 to 19 hours (carebastine) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C32H39NO2 |
| Molar mass | 469.669 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ebastine is a H1 antihistamine with low potential for causing drowsiness.
It does not penetrate the blood–brain barrier to a significant amount and thus combines an effective block of the H1 receptor in peripheral tissue with a low incidence of central side effects, i.e. seldom causing sedation or drowsiness.[1][2][3]
It was patented in 1983 by Almirall S.A and came into medical use in 1990.[4] The substance is often provided in micronised form due to poor water solubility.
Uses
Ebastine is a second-generation H1 receptor antagonist that is indicated mainly for allergic rhinitis and chronic idiopathic urticaria.[5] It is available in 10 and 20 mg tablets[6] and as fast-dissolving tablets,[7] as well as in pediatric syrup. It has a recommended flexible daily dose of 10 or 20 mg, depending on disease severity.
Data from over 8,000 patients in more than 40 clinical trials[failed verification] and studies[3][5][6][8][9][10] suggest efficacy of ebastine in the treatment of intermittent allergic rhinitis, persistent allergic rhinitis and other indications.
Safety
Ebastine has shown overall safety and tolerability profile with no cognitive/psychomotor impairment[6] and no sedation[6] worse than placebo,[2] and cardiac safety, that is, no QT prolongation.[6] The incidence of most commonly reported adverse events was comparable between the ebastine and placebo groups, which confirms that ebastine has a favourable safety profile.
While experiments in pregnant animals showed no risk for the unborn, no such data are available in humans. It is not known whether ebastine passes into the breast milk.
Pharmacokinetic profile
After oral administration, ebastine undergoes extensive first-pass metabolism by hepatic cytochrome P450 3A4 into its active carboxylic acid metabolite, carebastine. This conversion is practically complete.
Brand names
Ebastine is available in different formulations (tablets, fast dissolving tablets and syrup) and commercialized under different brand names around the world, Ebast, Ebatin, Ebatin Fast, Ebatrol, Atmos, Ebet, Ebastel FLAS, Kestine, KestineLIO, KestinLYO, EstivanLYO, Evastel Z,Tebast (SQUARE), Ebasten (ACI), etc.
See also
References
- ↑ "Neuroimaging of histamine H1-receptor occupancy in human brain by positron emission tomography (PET): a comparative study of ebastine, a second-generation antihistamine, and (+)-chlorpheniramine, a classical antihistamine". British Journal of Clinical Pharmacology 52 (5): 501–509. November 2001. doi:10.1046/j.1365-2125.2001.01471.x. PMID 11736858.

- ↑ 2.0 2.1 (in German) Arzneistoff-Profile. 4 (23 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. 2010. ISBN 978-3-7741-98-46-3.
- ↑ 3.0 3.1 "A 12-week, placebo-controlled study of the efficacy and safety of ebastine, 10 and 20 mg once daily, in the treatment of perennial allergic rhinitis. Multicentre Study Group". Allergy 54 (6): 562–568. June 1999. doi:10.1034/j.1398-9995.1999.00984.x. PMID 10435469.
- ↑ (in en) Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 549. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA549.
- ↑ 5.0 5.1 "A review of the second-generation antihistamine ebastine for the treatment of allergic disorders". Expert Opinion on Pharmacotherapy 5 (8): 1807–1813. August 2004. doi:10.1517/14656566.5.8.1807. PMID 15264995.
- ↑ 6.0 6.1 6.2 6.3 6.4 "Ebastine in allergic rhinitis and chronic idiopathic urticaria". Allergy 63 (Suppl 89): 1–20. December 2008. doi:10.1111/j.1398-9995.2008.01897.x. PMID 19032340.
- ↑ "Comparison of inhibition of cutaneous histamine reaction of ebastine fast-dissolving tablet (20 mg) versus desloratadine capsule (5 mg): a randomized, double-blind, double-dummy, placebo-controlled, three-period crossover study in healthy, nonatopic adults". Clinical Therapeutics 29 (5): 814–822. May 2007. doi:10.1016/j.clinthera.2007.05.001. PMID 17697901.
- ↑ "Meta-analysis of the efficacy of ebastine 20 mg compared to loratadine 10 mg and placebo in the symptomatic treatment of seasonal allergic rhinitis". International Archives of Allergy and Immunology 138 (4): 312–318. December 2005. doi:10.1159/000088869. PMID 16224195.
- ↑ "A comparison of ebastine 10 mg fast-dissolving tablet with oral desloratadine and placebo in inhibiting the cutaneous reaction to histamine in healthy adults". Clinical Drug Investigation 27 (7): 453–461. 2007. doi:10.2165/00044011-200727070-00002. PMID 17563125.
- ↑ "Comparison of ebastine to cetirizine in seasonal allergic rhinitis in adults". Annals of Allergy, Asthma & Immunology 76 (6): 507–512. June 1996. doi:10.1016/S1081-1206(10)63269-3. PMID 8673684.
External links
- "KESTINE Package Insert". South Africa n Electronic Package Inserts. 1997-10-24. http://home.intekom.com/pharm/lennon/kestine.html.
 |
