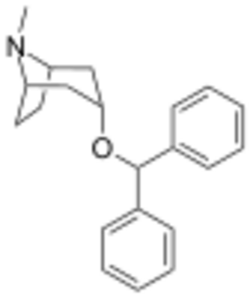
Chemistry:Benzatropine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cogentin, others |
| Other names | benzatropine (BAN UK), benztropine (USAN US) |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, IM, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Elimination half-life | 12–24 hours |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H25NO |
| Molar mass | 307.437 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Benzatropine (INN[1]), known as benztropine in the United States and Japan,[2] is a medication used to treat movement disorders like parkinsonism and dystonia, as well as extrapyramidal side effects of antipsychotics, including akathisia.[3] It is not useful for tardive dyskinesia.[3] It is taken by mouth or by injection into a vein or muscle.[3] Benefits are seen within two hours and last for up to ten hours.[4][5]
Common side effects include dry mouth, blurry vision, nausea, and constipation.[3] Serious side effect may include urinary retention, hallucinations, hyperthermia, and poor coordination.[3] It is unclear if use during pregnancy or breastfeeding is safe.[6] Benzatropine is an anticholinergic which works by blocking the activity of the muscarinic acetylcholine receptor.[3]
Benzatropine was approved for medical use in the United States in 1954.[3] It is available as a generic medication.[3] In 2020, it was the 229th most commonly prescribed medication in the United States, with more than 2 million prescriptions.[7][8] It is sold under the brand name Cogentin among others.[3]
Medical uses
Benzatropine is used to reduce extrapyramidal side effects of antipsychotic treatment. Benzatropine is also a second-line drug for the treatment of Parkinson's disease. It improves tremor, and may alleviate rigidity and bradykinesia.[9] Benzatropine is also sometimes used for the treatment of dystonia, a rare disorder that causes abnormal muscle contraction, resulting in twisting postures of limbs, trunk, or face.
Adverse effects
These are principally anticholinergic:
- Dry mouth
- Blurred vision
- Cognitive changes
- Drowsiness
- Constipation
- Urinary retention
- Tachycardia
- Anorexia
- Severe delirium and hallucinations (in overdose)
While some studies suggest that use of anticholinergics increases the risk of tardive dyskinesia (a long-term side effect of antipsychotics),[10][11] other studies have found no association between anticholinergic exposure and risk of developing tardive dyskinesia,[12] although symptoms may be worsened.[13]
Drugs that decrease cholinergic transmission may impair storage of new information into long-term memory. Anticholinergic agents can also impair time perception.[14]
Pharmacology
Benzatropine is a centrally acting anticholinergic/antihistamine agent. It is a selective M1 muscarinic acetylcholine receptor antagonist. Benzatropine partially blocks cholinergic activity in the basal ganglia and has also been shown to increase the availability of dopamine by blocking its reuptake and storage in central sites, and as a result, increasing dopaminergic activity. Animal studies have indicated that anticholinergic activity of benzatropine is approximately one-half that of atropine, while its antihistamine activity approaches that of mepyramine. Its anticholinergic effects have been established as therapeutically significant in the management of Parkinsonism. Benzatropine antagonizes the effect of acetylcholine, decreasing the imbalance between the neurotransmitters acetylcholine and dopamine, which may improve the symptoms of early Parkinson's disease.[15]
Benzatropine analogues are atypical dopamine reuptake inhibitors,[16] which might make them useful for people with akathisia secondary to antipsychotic therapy.[17]
Benzatropine also acts as a functional inhibitor of acid sphingomyelinase (FIASMA).[18]
Benzatropine has been also identified, by a high throughput screening approach, as a potent differentiating agent for oligodendrocytes, possibly working through M1 and M3 muscarinic receptors. In preclinical models for multiple sclerosis, benzatropine decreased clinical symptoms and enhanced re-myelination.[19]
Other animals
In veterinary medicine, benzatropine is used to treat priapism in stallions.[20]
Naming
Since 1959, benzatropine is the official international nonproprietary name of the medication under the INN scheme, the medication naming system coordinated by the World Health Organization; it is also the British Approved Name (BAN) given in the British Pharmacopoeia,[1][2] and has been the official nonproprietary name in Australia since 2015.[21] Regional variations of the "a" spelling are also used in French, Italian, Portuguese, and Spanish, as well as Latin (all medications are assigned a Latin name by WHO).[2]
"Benztropine" is the official United States Adopted Name (USAN), the medication naming system coordinated by the USAN Council, co-sponsored by the American Medical Association (AMA), the United States Pharmacopeial Convention (USP), and the American Pharmacists Association (APhA). It is also the Japanese Accepted Name (JAN)[22] and was used in Australia until 2015, when it was harmonized with the INN.[21]
Both names may be modified to account for the methanesulfonate salt as which the medication is formulated: the modified INN (INNm) and BAN (BANM) is benzatropine mesilate, while the modified USAN is benztropine mesylate.[23] The modified JAN is a hybrid form, benztropine mesilate.[22]
The misspelling benzotropine is also occasionally seen in the literature.
See also
- Gaboxadol
- Propantheline bromide
- Glycopyrrolate
- Oxybutynin
References
- ↑ 1.0 1.1 World Health Organization (December 1959). "International Non-Proprietary Names for Pharmaceutical Preparations). Recommended International Non-Proprietary Names (Rec. I.N.N.): List 3º". WHO Chronicle 13 (12): 464. https://www.who.int/medicines/publications/druginformation/innlists/RL03.pdf?ua=1. Retrieved 2020-12-01.
- ↑ 2.0 2.1 2.2 World Health Organization. "INN: Benzatropine". WHO MedNet. https://mednet-communities.net/inn/db/ViewINN.aspx?i=292.[yes|permanent dead link|dead link}}]
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 "Benztropine Mesylate Monograph for Professionals" (in en). American Society of Health-System Pharmacists. https://www.drugs.com/monograph/benztropine-mesylate.html.
- ↑ (in en) PNDR, Psychologists' Neuropsychotropic Drug Reference. Psychology Press. 1999. p. 47. ISBN 9780876309568. https://books.google.com/books?id=h_edjWaBilsC&pg=PA47.
- ↑ (in en) Drug Therapy in Nursing. Lippincott Williams & Wilkins. 2009. p. 197. ISBN 9780781765879. https://books.google.com/books?id=5zd_W_PUwvYC&pg=PA197.
- ↑ "Benztropine (Cogentin) Use During Pregnancy" (in en). https://www.drugs.com/pregnancy/benztropine.html.
- ↑ "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Benztropine - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Benztropine.
- ↑ "A controlled trial of amantadine in drug-induced extrapyramidal disorders". Archives of General Psychiatry 33 (5): 599–602. May 1976. doi:10.1001/archpsyc.1976.01770050055008. PMID 5066.
- ↑ "Tardive dyskinesia: prevalence and risk factors, 1959 to 1979". Archives of General Psychiatry 39 (4): 473–481. April 1982. doi:10.1001/archpsyc.1982.04290040069010. PMID 6121548.
- ↑ "Risk factors for tardive dyskinesia in a large population of youths and adults". Experimental and Clinical Psychopharmacology 9 (3): 285–296. August 2001. doi:10.1037/1064-1297.9.3.285. PMID 11534539.
- ↑ "Intermittent neuroleptic treatment and risk for tardive dyskinesia: Curaçao Extrapyramidal Syndromes Study III". The American Journal of Psychiatry 155 (4): 565–567. April 1998. doi:10.1176/ajp.155.4.565. PMID 9546009.
- ↑ "Tardive dyskinesia and anticholinergic drugs. A critical review of the literature". L'Encéphale 14 Spec No (Spec No): 233–239. September 1988. PMID 3063514.
- ↑ "Anticholinergic effects on memory: benztropine versus amantadine". Journal of Clinical Psychopharmacology 9 (3): 180–185. June 1989. doi:10.1097/00004714-198906000-00004. PMID 2661606.
- ↑ MIMS Australia Pty Ltd. MIMS.
- ↑ "Preclinical efficacy of N-substituted benztropine analogs as antagonists of methamphetamine self-administration in rats". The Journal of Pharmacology and Experimental Therapeutics 348 (1): 174–191. January 2014. doi:10.1124/jpet.113.208264. PMID 24194527.
- ↑ "A controlled comparison of the effects of propranolol, benztropine, and placebo on akathisia: an interim analysis". Psychopharmacology Bulletin 29 (2): 283–286. 1993. PMID 8290678.
- ↑ "Identification of novel functional inhibitors of acid sphingomyelinase". PLOS ONE 6 (8): e23852. 2011. doi:10.1371/journal.pone.0023852. PMID 21909365. Bibcode: 2011PLoSO...623852K.
- ↑ "A regenerative approach to the treatment of multiple sclerosis". Nature 502 (7471): 327–332. October 2013. doi:10.1038/nature12647. PMID 24107995. Bibcode: 2013Natur.502..327D.
- ↑ "Pharmacologic treatment of priapism in two horses". Journal of the American Veterinary Medical Association 199 (9): 1183–1184. November 1991. doi:10.2460/javma.1991.199.09.1183. PMID 1752772.
- ↑ 21.0 21.1 "Updating medicine ingredient names - list of affected ingredients". Therapeutic Goods Administration. 2015-11-23. https://www.tga.gov.au/updating-medicine-ingredient-names-list-affected-ingredients.
- ↑ 22.0 22.1 Compound D00778 at KEGG Pathway Database.
- ↑ Sweetman, Sean C., ed (2009). "Antiparkinsonian Drugs". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. pp. 797. ISBN 978-0-85369-840-1.
External links
- "Benzatropine". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/benzatropine.
 |
