Chemistry:Bromazine
 | |
| Clinical data | |
|---|---|
| Trade names | Ambodryl, Ambrodil, Deserol |
| Other names | Bromodiphenhydramine; Bromdiphenhydramine |
| MedlinePlus | a682065 |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | High |
| Protein binding | 96% |
| Metabolism | Mostly hepatic (CYP-mediated), also renal |
| Elimination half-life | 1 to 4 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H20BrNO |
| Molar mass | 334.257 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
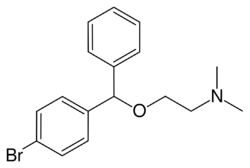
Bromazine, sold under the brand names Ambodryl, Ambrodil, and Deserol among others, also known as bromodiphenhydramine, is an antihistamine and anticholinergic medication of the ethanolamine class.[1][2][3][4][5] It is an analogue of diphenhydramine with a bromine substitution on one of the phenyl rings.[1][2]
Synthesis
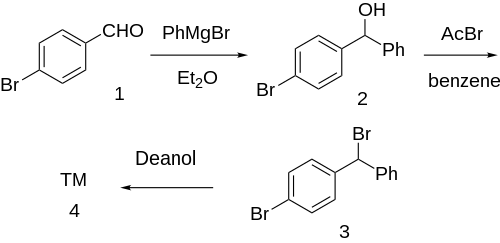
Grignard reaction between phenylmagnesium bromide and para-bromobenzaldehyde [1122-91-4] (1) gives p-bromobenzhydrol [29334-16-5] (2). Halogenation with acetyl bromide in benzene solvent gives p-bromo-benzhydrylbromide [18066-89-2] (3). Finally, etherification with deanol completed the synthesis of Bromazine (4).
Side effects
Continuous and/or cumulative use of anticholinergic medications, including first-generation antihistamines, is associated with higher risk for cognitive decline and dementia in elderly people.[8][9]
References
- ↑ 1.0 1.1 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 177–. ISBN 978-1-4757-2085-3. OCLC 1058412474. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA177.
- ↑ 2.0 2.1 Swiss Pharmaceutical Society (2000). Swiss Pharmaceutical Society. ed. Index Nominum 2000: International Drug Directory. Taylor & Francis. pp. 134–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA134.
- ↑ Physicians' Desk Reference (28th ed.). Oradell, NJ 07649: Medical Economics Company. 1974. pp. 1076, 1081.
- ↑ "Efficacy and safety of H1-antihistamines: an update". Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry 11 (3): 230–237. 2012. doi:10.2174/1871523011202030230. PMID 23173575.
- ↑ "A clinical comparison of carbinoxamine maleate, tripelennamine hydrochloride, and bromodiphenhydramine hydrochloride in treating allergic symptoms". Annals of Allergy 13 (3): 307–312. 1955. PMID 14377226.
- ↑ "Anti-inflammatory effects of two new methyl and morpholine derivatives of diphenhydramine on rats.". Medicinal Chemistry Research 21 (11): 3532–3540. November 2012. doi:10.1007/s00044-011-9891-y.
- ↑ Rieveschl Jr G, "Beta-dimethylamino-ethyl rho-halobenzhydryl ethers and their salts", US patent 2527963, issued 31 October 1950, assigned to Parke Davis & Co.
- ↑ "Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study". JAMA Internal Medicine 175 (3): 401–407. March 2015. doi:10.1001/jamainternmed.2014.7663. PMID 25621434.
- ↑ "Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study". Archives of Internal Medicine 169 (14): 1317–1324. July 2009. doi:10.1001/archinternmed.2009.229. PMID 19636034.
 |
