Biology:P2RX7
 Generic protein structure example |
| Part of a series on |
| Purinergic signalling |
|---|
 |
| Concepts |
| Membrane transporters |
P2X purinoceptor 7 is a protein that in humans is encoded by the P2RX7 gene.[1][2]
The product of this gene belongs to the family of purinoceptors for ATP. Multiple alternatively spliced variants which would encode different isoforms have been identified although some fit nonsense-mediated decay criteria.[3]
The receptor is found in the central and peripheral nervous systems, in microglia, in macrophages, in uterine endometrium, and in the retina.[4][5][6][7][8][9][10] The P2X7 receptor also serves as a pattern recognition receptor for extracellular ATP-mediated apoptotic cell death,[11][12][13] regulation of receptor trafficking,[14] mast cell degranulation,[15][16] and inflammation.[17][15][16][18] Regarding inflammation, P2X7 receptor induces the NLRP3 inflammasome in myeloid cells and leads to interleukin-1beta release[19].
Structure and kinetics
The P2X7 subunits can form homomeric receptors only with a typical P2X receptor structure.[20] The P2X7 receptor is a ligand-gated cation channel that opens in response to ATP binding and leads to cell depolarization. The P2X7 receptor requires higher levels of ATP than other P2X receptors; however, the response can be potentiated by reducing the concentration of divalent cations such as calcium or magnesium.[4][21] Continued binding leads to increased permeability to N-methyl-D-glucamine (NMDG+).[21] P2X7 receptors do not become desensitized readily and continued signaling leads to the aforementioned increased permeability and an increase in current amplitude.[21]
Pharmacology
Agonists
- P2X7 receptors respond to BzATP more readily than ATP.[21]
- ADP and AMP are weak agonists of P2X7 receptors, but a brief exposure to ATP can increase their effectiveness.[21]
- Glutathione has been proposed to act as a P2X7 receptor agonist when present at milimolar levels, inducing calcium transients and GABA release from retinal cells.[6][5]
Antagonists
- The P2X7 receptor current can be blocked by zinc, calcium, magnesium, and copper.[21]
- P2X7 receptors are sensitive to pyridoxalphosphate-6-azophenyl-2',4'-disulphonic acid (PPADS) and relatively insensitive to suramin, but the suramin analog, NF279, is much more effective.
- Oxidized ATP (OxATP) and Brilliant Blue G has also been used for blocking P2X7 in inflammation.[22][23]
- Other blockers include the large organic cations calmidazolium (a calmodulin antagonist) and KN-62 (a CaM kinase II antagonist).[21]
- JNJ-54175446 and JNJ-55308942 are selective antagonists
Receptor trafficking
In microglia, P2X7 receptors are found mostly on the cell surface.[24] Conserved cysteine residues located in the carboxyl terminus seem to be important for receptor trafficking to the cell membrane.[25] These receptors are upregulated in response to peripheral nerve injury.[26]
In melanocytic cells P2X7 gene expression may be regulated by MITF.[27]
Recruitment of pannexin
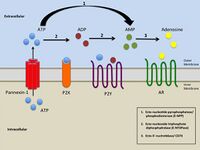
Activation of the P2X7 receptor by ATP leads to recruitment of pannexin pores[28] which allow small molecules such as ATP to leak out of cells. This allows further activation of purinergic receptors and physiological responses such a spreading cytoplasmic waves of calcium.[29] Moreover, this could be responsible for ATP-dependent lysis of macrophages through the formation of membrane pores permeable to larger molecules.
Clinical significance
Inflammation
On T cells activation of P2X7 receptors can activate the T cells or cause T cell differentiation, can affect T cell migration or (at high extracellular levels of ATP and/or NAD+) can induce cell death.[30] The CD38 enzyme on B lymphocytes and macrophages reduces extracellular NAD+, promoting the survival of T cells.[31]
Neuropathic pain
Microglial P2X7 receptors are thought to be involved in neuropathic pain because blockade or deletion of P2X7 receptors results in decreased responses to pain, as demonstrated in vivo.[32][33] Moreover, P2X7 receptor signaling increases the release of proinflammatory molecules such as IL-1β, IL-6, and TNF-α.[34][35][36] In addition, P2X7 receptors have been linked to increases in proinflammatory cytokines such as CXCL2 and CCL3.[37][38] P2X7 receptors are also linked to P2X4 receptors, which are also associated with neuropathic pain mediated by microglia.[24]
Osteoporosis
Mutations in this gene have been associated to low lumbar spine bone mineral density and accelerated bone loss in post-menopausal women.[39]
Diabetes
The ATP/P2X7R pathway may trigger T-cell attacks on the pancreas, rendering it unable to produce insulin. This autoimmune response may be an early mechanism by which the onset of diabetes is caused.[40][41]
Research
Possible link to hepatic fibrosis
One study in mice showed that blockade of P2X7 receptors attenuates onset of liver fibrosis.[42]
See also
- Purinergic receptor
- P2X receptor
References
- ↑ "The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA". The Journal of Biological Chemistry 272 (9): 5482–6. February 1997. doi:10.1074/jbc.272.9.5482. PMID 9038151.
- ↑ "Gene structure and chromosomal localization of the human P2X7 receptor". Receptors & Channels 5 (6): 347–54. Feb 1999. PMID 9826911.
- ↑ "Entrez Gene: P2RX7 purinergic receptor P2X, ligand-gated ion channel, 7". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=5027.
- ↑ 4.0 4.1 "P2X7 receptor large pore signaling in avian Müller glial cells". Journal of Bioenergetics and Biomembranes 49 (3): 215–229. June 2017. doi:10.1007/s10863-017-9717-9. PMID 28573491.
- ↑ 5.0 5.1 "7R activation on Müller glia". Neurogenesis 4 (1): e1283188. February 2017. doi:10.1080/23262133.2017.1283188. PMID 28229088.
- ↑ 6.0 6.1 "Glutathione-Induced Calcium Shifts in Chick Retinal Glial Cells". PLOS ONE 11 (4): e0153677. April 2016. doi:10.1371/journal.pone.0153677. PMID 27078878. Bibcode: 2016PLoSO..1153677F.
- ↑ "Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems". The Journal of Neuroscience 21 (18): 7143–52. September 2001. doi:10.1523/JNEUROSCI.21-18-07143.2001. PMID 11549725.
- ↑ "Tissue distribution of the P2X7 receptor". Neuropharmacology 36 (9): 1277–83. September 1997. doi:10.1016/S0028-3908(97)00140-8. PMID 9364482.
- ↑ "Distributional changes of purinergic receptor subtypes (P2X 1-7) in uterine epithelial cells during early pregnancy". The Histochemical Journal 32 (6): 365–72. June 2000. doi:10.1023/A:1004017714702. PMID 10943851.
- ↑ "Neuron-specific distribution of P2X7 purinergic receptors in the monkey retina". The Journal of Comparative Neurology 459 (3): 267–77. May 2003. doi:10.1002/cne.10608. PMID 12655509.
- ↑ Freitas (2019). "Interaction between cannabinoid and nucleotide systems as a new mechanism of signaling in retinal cell death". Neural Regeneration Research 14 (12): 2093–2094. doi:10.4103/1673-5374.262585. PMID 31397346.
- ↑ "Cannabinoids Induce Cell Death and Promote P2X7 Receptor Signaling in Retinal Glial Progenitors in Culture". Molecular Neurobiology 56 (9): 6472–6486. September 2019. doi:10.1007/s12035-019-1537-y. PMID 30838518.
- ↑ "Involvement of P2X4 receptor in P2X7 receptor-dependent cell death of mouse macrophages". Biochemical and Biophysical Research Communications 419 (2): 374–80. March 2012. doi:10.1016/j.bbrc.2012.01.156. PMID 22349510.
- ↑ "P2X7 receptors regulate multiple types of membrane trafficking responses and non-classical secretion pathways". Purinergic Signalling 5 (2): 163–73. June 2009. doi:10.1007/s11302-009-9132-8. PMID 19189228.
- ↑ 15.0 15.1 "New era for mucosal mast cells: their roles in inflammation, allergic immune responses and adjuvant development". Experimental & Molecular Medicine 46 (3): e83. March 2014. doi:10.1038/emm.2014.7. PMID 24626169.
- ↑ 16.0 16.1 "P2X7 receptors induce degranulation in human mast cells". Purinergic Signalling 12 (2): 235–46. June 2016. doi:10.1007/s11302-016-9497-4. PMID 26910735.
- ↑ "1-Aryl-1H- and 2-aryl-2H-1,2,3-triazole derivatives blockade P2X7 receptor in vitro and inflammatory response in vivo". European Journal of Medicinal Chemistry 139: 698–717. October 2017. doi:10.1016/j.ejmech.2017.08.034. PMID 28858765. http://www.sciencedirect.com/science/article/pii/S0223523417306372.
- ↑ "Immune Surveillance of the CNS following Infection and Injury". Trends in Immunology 36 (10): 637–650. October 2015. doi:10.1016/j.it.2015.08.002. PMID 26431941.
- ↑ Pelegrin, Pablo; Barroso-Gutierrez, Consuelo; Surprenant, Annmarie (2008-06-01). "P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage". Journal of Immunology (Baltimore, Md.: 1950) 180 (11): 7147–7157. doi:10.4049/jimmunol.180.11.7147. ISSN 0022-1767. PMID 18490713. https://pubmed.ncbi.nlm.nih.gov/18490713.
- ↑ "Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners". The Journal of Biological Chemistry 274 (10): 6653–9. March 1999. doi:10.1074/jbc.274.10.6653. PMID 10037762.
- ↑ 21.0 21.1 21.2 21.3 21.4 21.5 21.6 "Molecular physiology of P2X receptors". Physiological Reviews 82 (4): 1013–67. October 2002. doi:10.1152/physrev.00015.2002. PMID 12270951.
- ↑ "P2X7 receptor inhibition improves recovery after spinal cord injury". Nature Medicine 10 (8): 821–7. August 2004. doi:10.1038/nm1082. PMID 15258577.
- ↑ "Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury". Proceedings of the National Academy of Sciences of the United States of America 106 (30): 12489–93. July 2009. doi:10.1073/pnas.0902531106. PMID 19666625.
- ↑ 24.0 24.1 "Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells". The Journal of Biological Chemistry 284 (20): 13446–54. May 2009. doi:10.1074/jbc.M901255200. PMID 19304656.
- ↑ "Conserved ectodomain cysteines are essential for rat P2X7 receptor trafficking". Purinergic Signalling 8 (2): 317–25. June 2012. doi:10.1007/s11302-012-9291-x. PMID 22286664.
- ↑ "Induction of the P2X7 receptor in spinal microglia in a neuropathic pain model". Neuroscience Letters 504 (1): 57–61. October 2011. doi:10.1016/j.neulet.2011.08.058. PMID 21924325.
- ↑ "Novel MITF targets identified using a two-step DNA microarray strategy". Pigment Cell & Melanoma Research 21 (6): 665–76. December 2008. doi:10.1111/j.1755-148X.2008.00505.x. PMID 19067971.
- ↑ "P2X7 receptor-Pannexin1 complex: pharmacology and signaling". American Journal of Physiology. Cell Physiology 295 (3): C752-60. September 2008. doi:10.1152/ajpcell.00228.2008. PMID 18596211.
- ↑ "Adenosine signaling and function in glial cells". Cell Death and Differentiation 17 (7): 1071–82. July 2010. doi:10.1038/cdd.2009.131. PMID 19763139.
- ↑ "P2X7 Receptor at the Crossroads of T Cell Fate". International Journal of Molecular Sciences 21 (14): 4937. 2020. doi:10.3390/ijms21144937. PMID 32668623.
- ↑ "Complex roles of members of the ADP-ribosyl transferase super family in immune defences: looking beyond PARP1". Biochemical Pharmacology 84 (1): 11–20. 2012. doi:10.1016/j.bcp.2012.02.016. PMID 22402301.
- ↑ "A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat". The Journal of Pharmacology and Experimental Therapeutics 319 (3): 1376–85. December 2006. doi:10.1124/jpet.106.111559. PMID 16982702.
- ↑ "Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain". Pain 114 (3): 386–96. April 2005. doi:10.1016/j.pain.2005.01.002. PMID 15777864.
- ↑ "P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide". The Journal of Neuroscience 30 (2): 573–82. January 2010. doi:10.1523/JNEUROSCI.3295-09.2010. PMID 20071520.
- ↑ "Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5". Journal of Neurochemistry 78 (6): 1339–49. September 2001. doi:10.1046/j.1471-4159.2001.00514.x. PMID 11579142.
- ↑ "Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia". Journal of Neurochemistry 75 (3): 965–72. September 2000. doi:10.1046/j.1471-4159.2000.0750965.x. PMID 10936177.
- ↑ "P2X7 receptor activation induces CXCL2 production in microglia through NFAT and PKC/MAPK pathways". Journal of Neurochemistry 114 (3): 810–9. August 2010. doi:10.1111/j.1471-4159.2010.06809.x. PMID 20477948.
- ↑ "Activation of P2X7 receptors induces CCL3 production in microglial cells through transcription factor NFAT". Journal of Neurochemistry 108 (1): 115–25. January 2009. doi:10.1111/j.1471-4159.2008.05744.x. PMID 19014371.
- ↑ "Polymorphisms in the P2X7 receptor gene are associated with low lumbar spine bone mineral density and accelerated bone loss in post-menopausal women". European Journal of Human Genetics 20 (5): 559–64. May 2012. doi:10.1038/ejhg.2011.245. PMID 22234152.
- ↑ "Silencing immune attacks in type 1 diabetes". June 10, 2013. http://vectorblog.org/2013/06/silencing-immune-attacks-in-type-1-diabetes-without/#more-8597.
- ↑ "Boston Children's Hospital Finds Root Cause of Diabetes". June 13, 2013. http://www.bostonmagazine.com/health/blog/2013/06/13/boston-childrens-hospital-found-the-root-cause-of-diabetes/.
- ↑ "P2X7 blockade attenuates mouse liver fibrosis". Molecular Medicine Reports 9 (1): 57–62. January 2014. doi:10.3892/mmr.2013.1807. PMID 24247209. http://www.spandidos-publications.com/mmr/9/1/57.
Further reading
- "P2 receptors in bone--modulation of osteoclast formation and activity via P2X7 activation". Critical Reviews in Eukaryotic Gene Expression 13 (2–4): 237–42. 2003. doi:10.1615/CritRevEukaryotGeneExpr.v13.i24.150. PMID 14696970.
- "Blockade of the pore-forming P2X7 receptor inhibits formation of multinucleated human osteoclasts in vitro". Calcified Tissue International 73 (4): 361–9. October 2003. doi:10.1007/s00223-002-2098-y. PMID 12874700.
- "Extracellular nucleotide signaling: a mechanism for integrating local and systemic responses in the activation of bone remodeling". Bone 28 (5): 507–12. May 2001. doi:10.1016/S8756-3282(01)00430-6. PMID 11344050.
- "Expression of a P2X7 receptor by a subpopulation of human osteoblasts". Journal of Bone and Mineral Research 16 (5): 846–56. May 2001. doi:10.1359/jbmr.2001.16.5.846. PMID 11341329.
- "Multinucleated osteoclast formation in vivo and in vitro by P2X7 receptor-deficient mice". Critical Reviews in Eukaryotic Gene Expression 13 (2–4): 243–53. 2003. doi:10.1615/CritRevEukaryotGeneExpr.v13.i24.160. PMID 14696971.
- "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene 138 (1–2): 171–4. January 1994. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene 200 (1–2): 149–56. October 1997. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- "A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor". The Journal of Biological Chemistry 276 (14): 11135–42. April 2001. doi:10.1074/jbc.M010353200. PMID 11150303.
- "Proteomic and functional evidence for a P2X7 receptor signalling complex". The EMBO Journal 20 (22): 6347–58. November 2001. doi:10.1093/emboj/20.22.6347. PMID 11707406.
- "Point mutations confer loss of ATP-induced human P2X(7) receptor function". FEBS Letters 512 (1–3): 43–6. February 2002. doi:10.1016/S0014-5793(01)03311-7. PMID 11852049.
- "A loss-of-function polymorphic mutation in the cytolytic P2X7 receptor gene and chronic lymphocytic leukaemia: a molecular study". Lancet 359 (9312): 1114–9. March 2002. doi:10.1016/S0140-6736(02)08156-4. PMID 11943260.
- "Epithelial membrane proteins induce membrane blebbing and interact with the P2X7 receptor C terminus". The Journal of Biological Chemistry 277 (37): 34017–23. September 2002. doi:10.1074/jbc.M205120200. PMID 12107182.
- "An ATP-gated ion channel at the cell nucleus". Nature 420 (6911): 42. November 2002. doi:10.1038/420042a. PMID 12422208.
- "Extracellular adenosine 5'-triphosphate induces a loss of CD23 from human dendritic cells via activation of P2X7 receptors". International Immunology 14 (12): 1415–21. December 2002. doi:10.1093/intimm/dxf111. PMID 12456589.
- "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proceedings of the National Academy of Sciences of the United States of America 99 (26): 16899–903. December 2002. doi:10.1073/pnas.242603899. PMID 12477932. Bibcode: 2002PNAS...9916899M.
- "An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor". The Journal of Biological Chemistry 278 (19): 17108–13. May 2003. doi:10.1074/jbc.M212759200. PMID 12586825.
- "Specific detection of non-functional human P2X(7) receptors in HEK293 cells and B-lymphocytes". FEBS Letters 538 (1–3): 159–62. March 2003. doi:10.1016/S0014-5793(03)00172-8. PMID 12633871.
- "P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1 beta release". Journal of Immunology 170 (11): 5728–38. June 2003. doi:10.4049/jimmunol.170.11.5728. PMID 12759456.
- "Purinergic receptors are part of a functional signaling system for proliferation and differentiation of human epidermal keratinocytes". The Journal of Investigative Dermatology 120 (6): 1007–15. June 2003. doi:10.1046/j.1523-1747.2003.12261.x. PMID 12787128.
- "Mutation of a dibasic amino acid motif within the C terminus of the P2X7 nucleotide receptor results in trafficking defects and impaired function". Journal of Immunology 171 (3): 1304–11. August 2003. doi:10.4049/jimmunol.171.3.1304. PMID 12874219.
External links
- P2RX7+protein,+human at the US National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
 |
