Chemistry:Molybdenum difluoride dioxide
| |
| Names | |
|---|---|
| Other names
molybdenum dioxide difluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| F2MoO2 | |
| Molar mass | 165.94 g·mol−1 |
| Appearance | white solid |
| Density | 3.82 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
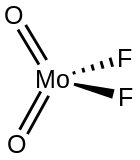
Molybdenum difluoride dioxide is the inorganic compound with the formula MoF2O2. It is a white, diamagnetic, volatile solid.[1]
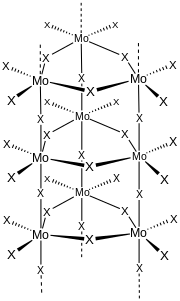
Structure
Gaseous molybdenum difluoride dioxide is a tetrahedral molecule.[2] According to X-ray crystallography, the solid is a coordination polymer consisting of trigonal primatic chains of made by linking Mo
3F
6O
6 monomers. The fluoride and oxide positions are disordered.[3] A similar motif is adopted by titanium tetrafluoride.
Synthesis and reactions
The compound can be obtained by thermal decomposition of the dioxotetrafluoride, which in turn is obtained from sodium molybdate:[3]
- Na
2MoO
4 + 4 HF → Na
2MoF
4O
2 + 2 H
2O
Heating sodium dioxotetrafluoride to 400 °C gives monomeric difluoride dioxide, which polymerizes upon condensation:
- Na
2MoF
4O
2 → 2 NaF + MoF
2O
2
The compound also arises by hydrolysis of molybdenum oxytetrafluoride:
- MoF
4O + H
2O → 2 HF + MoF
2O
2
The compound dissolves in dimethylformamide to give a bis(adduct):[4]
- MoF
2O
2 + 2 OC(H)N(CH
3)
2 → MoF
2O
2(OC(H)N(CH
3)
2)
2
Further reading
- Edwards, A. J.; Steventon, B. R. (1968). "Fluoride crystal structures. Part II. Molybdenum oxide tetrafluoride". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 2503. doi:10.1039/j19680002503.
- Turnbull, Douglas; Chaudhary, Praveen; Leenstra, Dakota; Hazendonk, Paul; Wetmore, Stacey D.; Gerken, Michael (2020). "Reactions of Molybdenum and Tungsten Oxide Tetrafluoride with Sulfur(IV) Lewis Bases: Structure and Bonding in [WOF4]4, MOF4(OSO), and [SF3][M2O2F9] (M = Mo, W)". Inorganic Chemistry 59 (23): 17544–17554. doi:10.1021/acs.inorgchem.0c02783. PMID 33200611.
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 1023. ISBN 978-0-08-037941-8.
- ↑ Ward, Brian G.; Stafford, Fred E. (1968). "Synthesis and Structure of Four- and Five-Coordinated Gaseous Oxohalides of Molybdenum(VI) and Tungsten(VI)". Inorganic Chemistry 7 (12): 2569–2573. doi:10.1021/ic50070a020.
- ↑ Jump up to: 3.0 3.1 Shorafa, Hashem; Ficicioglu, Halil; Tamadon, Farhad; Girgsdies, Frank; Seppelt, Konrad (2010). "Molybdenum Difluoride Dioxide, MoO2F2". Inorganic Chemistry 49 (9): 4263–4267. doi:10.1021/ic1000864. PMID 20380384.
- ↑ Benjamin, Sophie L.; Levason, William; Reid, Gillian (2013). "Medium and high oxidation state metal/Non-metal fluoride and oxide–fluoride complexes with neutral donor ligands". Chem. Soc. Rev. 42 (4): 1460–1499. doi:10.1039/C2CS35263J. PMID 23014811.
 |