Unsolved:Hypothetical types of biochemistry
"The existence of lakes of liquid hydrocarbons on Titan opens up the possibility for solvents and energy sources that are alternatives to those in our biosphere and that might support novel life forms altogether different from those on Earth."—NASA Astrobiology Roadmap 2008[1]
Hypothetical types of biochemistry are forms of biochemistry agreed to be scientifically viable but not proven to exist at this time.[2] The kinds of living organisms currently known on Earth all use carbon compounds for basic structural and metabolic functions, water as a solvent, and DNA or RNA to define and control their form. If life exists on other planets or moons it may be chemically similar, though it is also possible that there are organisms with quite different chemistries[3] – for instance, involving other classes of carbon compounds, compounds of another element, or another solvent in place of water.
The possibility of life-forms being based on "alternative" biochemistries is the topic of an ongoing scientific discussion, informed by what is known about extraterrestrial environments and about the chemical behaviour of various elements and compounds. It is of interest in synthetic biology and is also a common subject in science fiction.
The element silicon has been much discussed as a hypothetical alternative to carbon. Silicon is in the same group as carbon on the periodic table and, like carbon, it is tetravalent. Hypothetical alternatives to water include ammonia, which, like water, is a polar molecule, and cosmically abundant; and non-polar hydrocarbon solvents such as methane and ethane, which are known to exist in liquid form on the surface of Titan.
Overview
| Type | Basis | Synopsis | Remarks |
|---|---|---|---|
| Alternative-chirality biomolecules | Alternative biochemistry | Mirror image biochemistry | Perhaps the least unusual alternative biochemistry would be one with differing chirality of its biomolecules. In known Earth-based life, amino acids are almost universally of the L form and sugars are of the D form. Molecules using D amino acids or L sugars are possible, though would be incompatible with organisms using the opposing chirality molecules. Gram-positive bacteria incorporate D Alanine into their Peptidoglycan layer, created through the actions of Racemases[4] |
| Ammonia biochemistry | Non-water solvents | Ammonia-based life | Ammonia is relatively abundant in the universe and has chemical similarities to water. The possible role of liquid ammonia as an alternative solvent for life is an idea that goes back at least to 1954, when J. B. S. Haldane raised the topic at a symposium about life's origin. |
| Arsenic biochemistry | Alternative biochemistry | Arsenic-based life | Arsenic, which is chemically similar to phosphorus, while poisonous for most life forms on Earth, is incorporated into the biochemistry of some organisms. |
| Borane biochemistry (Organoboron chemistry) | Alternative biochemistry | Boranes-based life | Boranes are dangerously explosive in Earth's atmosphere, but would be more stable in a reducing environment. Boron, however, is exceedingly rare in the universe in comparison to its neighbours carbon, nitrogen, and oxygen. On the other hand, structures containing alternating boron and nitrogen atoms share some properties with hydrocarbons. |
| Cosmic necklace-based biology | Nonplanetary life | Non-chemical life | In 2020, Luis A. Anchordoqu and Eugene M. Chudnovsky hypothesized that life composed of magnetic semipoles connected by cosmic strings could evolve inside stars.[5] |
| Dusty plasma-based biology | Nonplanetary life | Non-chemical life | In 2007, Vadim N. Tsytovich and colleagues proposed that lifelike behaviors could be exhibited by dust particles suspended in a plasma, under conditions that might exist in space.[6] |
| Extremophiles | Alternative environment | Life in variable environments | It would be biochemically possible to sustain life in environments that are only periodically consistent with life as we know it. |
| Heteropoly acid biochemistry | Alternative biochemistry | Heteropoly acid-based life | Various metals can form complex structures with oxygen, such as heteropoly acids. |
| Hydrogen fluoride biochemistry | Non-water solvents | Hydrogen fluoride-based life | Hydrogen fluoride has been considered as a possible solvent for life by scientists such as Peter Sneath. |
| Hydrogen sulfide biochemistry | Non-water solvents | Hydrogen sulfide-based life | Hydrogen sulfide is a chemical analog of water, but is less polar and a weaker inorganic solvent. |
| Methane biochemistry (Azotosome) | Non-water solvents | Methane-based life | Methane (CH4) is relatively abundant in the solar system and the universe, and is known to exist in liquid form on Titan, the largest moon of Saturn. Though highly unlikely, it is considered to be possible for Titan to harbor life. If so, it will most likely be Methane-based life. |
| Non-green photosynthesizers | Other speculations | Alternate plant life | Physicists have noted that, although photosynthesis on Earth generally involves green plants, a variety of other-colored plants could also support photosynthesis, essential for most life on Earth, and that other colors might be preferred in places that receive a different mix of stellar radiation than Earth. In particular, retinal is capable of, and has been observed to, perform photosynthesis.[7] Bacteria capable of photosynthesis are known as microbial rhodopsins. A plant or creature that uses retinal photosynthesis is always purple. |
| Shadow biosphere | Alternative environment | A hidden life biosphere on Earth | A shadow biosphere is a hypothetical microbial biosphere of Earth that uses radically different biochemical and molecular processes than currently known life. |
| Silicon biochemistry (Organosilicon) | Alternative biochemistry | Silicon-based life | Like carbon, silicon can create molecules that are sufficiently large to carry biological information; however, the scope of possible silicon chemistry is far more limited than that of carbon. |
| Silicon dioxide biochemistry | Non-water solvents | Silicon dioxide-based life | Gerald Feinberg and Robert Shapiro have suggested that molten silicate rock could serve as a liquid medium for organisms with a chemistry based on silicon, oxygen, and other elements such as aluminium. |
| Sulfur biochemistry | Alternative biochemistry | Sulfur-based life | The biological use of sulfur as an alternative to carbon is purely hypothetical, especially because sulfur usually forms only linear chains rather than branched ones. |
| Alternative nucleic acids | Alternative biochemistry | Different genetic storage |
Shadow biosphere

A shadow biosphere is a hypothetical microbial biosphere of Earth that uses radically different biochemical and molecular processes than currently known life.[8][9] Although life on Earth is relatively well-studied, the shadow biosphere may still remain unnoticed because the exploration of the microbial world targets primarily the biochemistry of the macro-organisms.
Alternative-chirality biomolecules
Perhaps the least unusual alternative biochemistry would be one with differing chirality of its biomolecules. In known Earth-based life, amino acids are almost universally of the L form and sugars are of the D form. Molecules using D amino acids or L sugars may be possible; molecules of such a chirality, however, would be incompatible with organisms using the opposing chirality molecules. Amino acids whose chirality is opposite to the norm are found on Earth, and these substances are generally thought to result from decay of organisms of normal chirality. However, physicist Paul Davies speculates that some of them might be products of "anti-chiral" life.[10]
It is questionable, however, whether such a biochemistry would be truly alien. Although it would certainly be an alternative stereochemistry, molecules that are overwhelmingly found in one enantiomer throughout the vast majority of organisms can nonetheless often be found in another enantiomer in different (often basal) organisms such as in comparisons between members of Archaea and other domains,[citation needed] making it an open topic whether an alternative stereochemistry is truly novel.
Non-carbon-based biochemistries
On Earth, all known living things have a carbon-based structure and system. Scientists have speculated about the pros and cons of using atoms other than carbon to form the molecular structures necessary for life, but no one has proposed a theory employing such atoms to form all the necessary structures. However, as Carl Sagan argued, it is very difficult to be certain whether a statement that applies to all life on Earth will turn out to apply to all life throughout the universe.[11] Sagan used the term "carbon chauvinism" for such an assumption.[12] He regarded silicon and germanium as conceivable alternatives to carbon[12] (other plausible elements include but are not limited to palladium and titanium); but, on the other hand, he noted that carbon does seem more chemically versatile and is more abundant in the cosmos).[13] Norman Horowitz devised the experiments to determine whether life might exist on Mars that were carried out by the Viking Lander of 1976, the first U.S. mission to successfully land an uncrewed probe on the surface of Mars. Horowitz argued that the great versatility of the carbon atom makes it the element most likely to provide solutions, even exotic solutions, to the problems of survival on other planets.[14] He considered that there was only a remote possibility that non-carbon life forms could exist with genetic information systems capable of self-replication and the ability to evolve and adapt.
Silicon biochemistry
The silicon atom has been much discussed as the basis for an alternative biochemical system, because silicon has many chemical properties similar to those of carbon and is in the same group of the periodic table, the carbon group. Like carbon, silicon can create molecules that are sufficiently large to carry biological information.[15]
However, silicon has several drawbacks as an alternative to carbon. Silicon, unlike carbon, lacks the ability to form chemical bonds with diverse types of atoms as is necessary for the chemical versatility required for metabolism, and yet this precise inability is what makes silicon less susceptible to bond with all sorts of impurities from which carbon, in comparison, is not shielded. Elements creating organic functional groups with carbon include hydrogen, oxygen, nitrogen, phosphorus, sulfur, and metals such as iron, magnesium, and zinc. Silicon, on the other hand, interacts with very few other types of atoms.[15] Moreover, where it does interact with other atoms, silicon creates molecules that have been described as "monotonous compared with the combinatorial universe of organic macromolecules".[15] This is because silicon atoms are much bigger, having a larger mass and atomic radius, and so have difficulty forming double and triple bonds (the double-bonded carbon is part of the carbonyl group, a fundamental motif of carbon-based bio-organic chemistry).
Silanes, which are chemical compounds of hydrogen and silicon that are analogous to the alkane hydrocarbons, are highly reactive with water, and long-chain silanes spontaneously decompose. Molecules incorporating polymers of alternating silicon and oxygen atoms instead of direct bonds between silicon, known collectively as silicones, are much more stable. It has been suggested that silicone-based chemicals would be more stable than equivalent hydrocarbons in a sulfuric-acid-rich environment, as is found in some extraterrestrial locations.[16]
Of the varieties of molecules identified in the interstellar medium (As of 1998), 84 are based on carbon, while only 8 are based on silicon.[17] Moreover, of those 8 compounds, 4 also include carbon within them. The cosmic abundance of carbon to silicon is roughly 10 to 1. This may suggest a greater variety of complex carbon compounds throughout the cosmos, providing less of a foundation on which to build silicon-based biologies, at least under the conditions prevalent on the surface of planets. Also, even though Earth and other terrestrial planets are exceptionally silicon-rich and carbon-poor (the relative abundance of silicon to carbon in Earth's crust is roughly 925:1), terrestrial life is carbon-based. The fact that carbon is used instead of silicon may be evidence that silicon is poorly suited for biochemistry on Earth-like planets. Reasons for this may be that silicon is less versatile than carbon in forming compounds, that the compounds formed by silicon are unstable, and that it blocks the flow of heat.[18]
Even so, biogenic silica is used by some Earth life, such as the silicate skeletal structure of diatoms. According to the clay hypothesis of A. G. Cairns-Smith, silicate minerals in water played a crucial role in abiogenesis: they replicated their crystal structures, interacted with carbon compounds, and were the precursors of carbon-based life.[19][20]
Although not observed in nature, carbon–silicon bonds have been added to biochemistry by using directed evolution (artificial selection). A heme containing cytochrome c protein from Rhodothermus marinus has been engineered using directed evolution to catalyze the formation of new carbon–silicon bonds between hydrosilanes and diazo compounds.[21]
Silicon compounds may possibly be biologically useful under temperatures or pressures different from the surface of a terrestrial planet, either in conjunction with or in a role less directly analogous to carbon. Polysilanols, the silicon compounds corresponding to sugars, are soluble in liquid nitrogen, suggesting that they could play a role in very-low-temperature biochemistry.[22][23]
Other exotic element-based biochemistries
- Boranes are dangerously explosive in Earth's atmosphere, but would be more stable in a reducing atmosphere. However, boron's low cosmic abundance makes it less likely as a base for life than carbon.
- Various metals, together with oxygen, can form very complex and thermally stable structures rivaling those of organic compounds;[citation needed] the heteropoly acids are one such family. Some metal oxides are also similar to carbon in their ability to form both nanotube structures and diamond-like crystals (such as cubic zirconia). Titanium, aluminium, magnesium, and iron are all more abundant in the Earth's crust than carbon. Metal-oxide-based life could therefore be a possibility under certain conditions, including those (such as high temperatures) at which carbon-based life would be unlikely. The Cronin group at Glasgow University reported self-assembly of tungsten polyoxometalates into cell-like spheres.[24] By modifying their metal oxide content, the spheres can acquire holes that act as porous membrane, selectively allowing chemicals in and out of the sphere according to size.[24]
- Sulfur is also able to form long-chain molecules, but suffers from the same high-reactivity problems as phosphorus and silanes. The biological use of sulfur as an alternative to carbon is purely hypothetical, especially because sulfur usually forms only linear chains rather than branched ones. (The biological use of sulfur as an electron acceptor is widespread and can be traced back 3.5 billion years on Earth, thus predating the use of molecular oxygen.[25] Sulfur-reducing bacteria can utilize elemental sulfur instead of oxygen, reducing sulfur to hydrogen sulfide.)
Arsenic as an alternative to phosphorus
Arsenic, which is chemically similar to phosphorus, while poisonous for most life forms on Earth, is incorporated into the biochemistry of some organisms.[26] Some marine algae incorporate arsenic into complex organic molecules such as arsenosugars and arsenobetaines. Fungi and bacteria can produce volatile methylated arsenic compounds. Arsenate reduction and arsenite oxidation have been observed in microbes (Chrysiogenes arsenatis).[27] Additionally, some prokaryotes can use arsenate as a terminal electron acceptor during anaerobic growth and some can utilize arsenite as an electron donor to generate energy.
It has been speculated that the earliest life forms on Earth may have used arsenic biochemistry in place of phosphorus in the structure of their DNA.[28] A common objection to this scenario is that arsenate esters are so much less stable to hydrolysis than corresponding phosphate esters that arsenic is poorly suited for this function.[29]
The authors of a 2010 geomicrobiology study, supported in part by NASA, have postulated that a bacterium, named GFAJ-1, collected in the sediments of Mono Lake in eastern California , can employ such 'arsenic DNA' when cultured without phosphorus.[30][31] They proposed that the bacterium may employ high levels of poly-β-hydroxybutyrate or other means to reduce the effective concentration of water and stabilize its arsenate esters.[31] This claim was heavily criticized almost immediately after publication for the perceived lack of appropriate controls.[32][33] Science writer Carl Zimmer contacted several scientists for an assessment: "I reached out to a dozen experts ... Almost unanimously, they think the NASA scientists have failed to make their case".[34] Other authors were unable to reproduce their results and showed that the study had issues with phosphate contamination, suggesting that the low amounts present could sustain extremophile lifeforms.[35] Alternatively, it was suggested that GFAJ-1 cells grow by recycling phosphate from degraded ribosomes, rather than by replacing it with arsenate.[36]
Non-water solvents
In addition to carbon compounds, all currently known terrestrial life also requires water as a solvent. This has led to discussions about whether water is the only liquid capable of filling that role. The idea that an extraterrestrial life-form might be based on a solvent other than water has been taken seriously in recent scientific literature by the biochemist Steven Benner,[37] and by the astrobiological committee chaired by John A. Baross.[38] Solvents discussed by the Baross committee include ammonia,[39] sulfuric acid,[40] formamide,[41] hydrocarbons,[41] and (at temperatures much lower than Earth's) liquid nitrogen, or hydrogen in the form of a supercritical fluid.[42]
Water as a solvent limits the forms biochemistry can take. For example, Steven Benner, proposes the polyelectrolyte theory of the gene that claims that for a genetic biopolymer such as, DNA, to function in water, it requires repeated ionic charges.[43] If water is not not required for life, these limits on genetic biopolymers are removed.
Carl Sagan once described himself as both a carbon chauvinist and a water chauvinist;[44] however, on another occasion he said that he was a carbon chauvinist but "not that much of a water chauvinist".[45] He speculated on hydrocarbons,[45]:11 hydrofluoric acid,[46] and ammonia[45][46] as possible alternatives to water.
Some of the properties of water that are important for life processes include:
- A complexity which leads to a large number of permutations of possible reaction paths including acid–base chemistry, H+ cations, OH− anions, hydrogen bonding, van der Waals bonding, dipole–dipole and other polar interactions, aqueous solvent cages, and hydrolysis. This complexity offers a large number of pathways for evolution to produce life, many other solvents[which?] have dramatically fewer possible reactions, which severely limits evolution.
- Thermodynamic stability: the free energy of formation of liquid water is low enough (−237.24 kJ/mol) that water undergoes few reactions. Other solvents are highly reactive, particularly with oxygen.
- Water does not combust in oxygen because it is already the combustion product of hydrogen with oxygen. Most alternative solvents are not stable in an oxygen-rich atmosphere, so it is highly unlikely that those liquids could support aerobic life.
- A large temperature range over which it is liquid.
- High solubility of oxygen and carbon dioxide at room temperature supporting the evolution of aerobic aquatic plant and animal life.
- A high heat capacity (leading to higher environmental temperature stability).
- Water is a room-temperature liquid leading to a large population of quantum transition states required to overcome reaction barriers. Cryogenic liquids (such as liquid methane) have exponentially lower transition state populations which are needed for life based on chemical reactions. This leads to chemical reaction rates which may be so slow as to preclude the development of any life based on chemical reactions.[citation needed]
- Spectroscopic transparency allowing solar radiation to penetrate several meters into the liquid (or solid), greatly aiding the evolution of aquatic life.
- A large heat of vaporization leading to stable lakes and oceans.
- The ability to dissolve a wide variety of compounds.
- The solid (ice) has lower density than the liquid, so ice floats on the liquid. This is why bodies of water freeze over but do not freeze solid (from the bottom up). If ice were denser than liquid water (as is true for nearly all other compounds), then large bodies of liquid would slowly freeze solid, which would not be conducive to the formation of life.
Water as a compound is cosmically abundant, although much of it is in the form of vapor or ice. Subsurface liquid water is considered likely or possible on several of the outer moons: Enceladus (where geysers have been observed), Europa, Titan, and Ganymede. Earth and Titan are the only worlds currently known to have stable bodies of liquid on their surfaces.
Not all properties of water are necessarily advantageous for life, however.[47] For instance, water ice has a high albedo,[47] meaning that it reflects a significant quantity of light and heat from the Sun. During ice ages, as reflective ice builds up over the surface of the water, the effects of global cooling are increased.[47]
There are some properties that make certain compounds and elements much more favorable than others as solvents in a successful biosphere. The solvent must be able to exist in liquid equilibrium over a range of temperatures the planetary object would normally encounter. Because boiling points vary with the pressure, the question tends not to be does the prospective solvent remain liquid, but at what pressure. For example, hydrogen cyanide has a narrow liquid-phase temperature range at 1 atmosphere, but in an atmosphere with the pressure of Venus, with 92 bars (91 atm) of pressure, it can indeed exist in liquid form over a wide temperature range.
Ammonia
The ammonia molecule (NH3), like the water molecule, is abundant in the universe, being a compound of hydrogen (the simplest and most common element) with another very common element, nitrogen.[48] The possible role of liquid ammonia as an alternative solvent for life is an idea that goes back at least to 1954, when J. B. S. Haldane raised the topic at a symposium about life's origin.[49]
Numerous chemical reactions are possible in an ammonia solution, and liquid ammonia has chemical similarities with water.[48][50] Ammonia can dissolve most organic molecules at least as well as water does and, in addition, it is capable of dissolving many elemental metals. Haldane made the point that various common water-related organic compounds have ammonia-related analogs; for instance the ammonia-related amine group (−NH2) is analogous to the water-related hydroxyl group (−OH).[50]
Ammonia, like water, can either accept or donate an H+ ion. When ammonia accepts an H+, it forms the ammonium cation (NH4+), analogous to hydronium (H3O+). When it donates an H+ ion, it forms the amide anion (NH2−), analogous to the hydroxide anion (OH−).[39] Compared to water, however, ammonia is more inclined to accept an H+ ion, and less inclined to donate one; it is a stronger nucleophile.[39] Ammonia added to water functions as Arrhenius base: it increases the concentration of the anion hydroxide. Conversely, using a solvent system definition of acidity and basicity, water added to liquid ammonia functions as an acid, because it increases the concentration of the cation ammonium.[50] The carbonyl group (C=O), which is much used in terrestrial biochemistry, would not be stable in ammonia solution, but the analogous imine group (C=NH) could be used instead.[39]
However, ammonia has some problems as a basis for life. The hydrogen bonds between ammonia molecules are weaker than those in water, causing ammonia's heat of vaporization to be half that of water, its surface tension to be a third, and reducing its ability to concentrate non-polar molecules through a hydrophobic effect. Gerald Feinberg and Robert Shapiro have questioned whether ammonia could hold prebiotic molecules together well enough to allow the emergence of a self-reproducing system.[51] Ammonia is also flammable in oxygen and could not exist sustainably in an environment suitable for aerobic metabolism.[52]
A biosphere based on ammonia would likely exist at temperatures or air pressures that are extremely unusual in relation to life on Earth. Life on Earth usually exists within the melting point and boiling point of water, at a pressure designated as normal pressure, and between 0 and 100 °C (273 and 373 K). When also held to normal pressure, ammonia's melting and boiling points are −78 °C (195 K) and −33 °C (240 K) respectively. Because chemical reactions generally proceed more slowly at lower temperatures, ammonia-based life existing in this set of conditions might metabolize more slowly and evolve more slowly than life on Earth.[52] On the other hand, lower temperatures could also enable living systems to use chemical species that would be too unstable at Earth temperatures to be useful.[48]
Another set of conditions where ammonia is liquid at Earth-like temperatures would involve it being at a much higher pressure. For example, at 60 atm ammonia melts at −77 °C (196 K) and boils at 98 °C (371 K).[39]
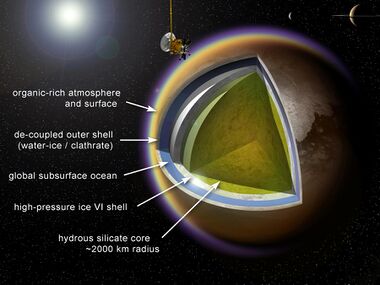
Ammonia and ammonia–water mixtures remain liquid at temperatures far below the freezing point of pure water, so such biochemistries might be well suited to planets and moons orbiting outside the water-based habitability zone. Such conditions could exist, for example, under the surface of Saturn's largest moon Titan.[53]
Methane and other hydrocarbons
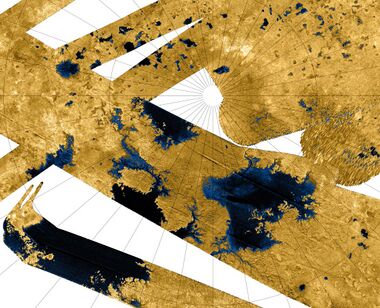
Methane (CH4) is a simple hydrocarbon: that is, a compound of two of the most common elements in the cosmos: hydrogen and carbon. It has a cosmic abundance comparable with ammonia.[48] Hydrocarbons could act as a solvent over a wide range of temperatures, but would lack polarity. Isaac Asimov, the biochemist and science fiction writer, suggested in 1981 that poly-lipids could form a substitute for proteins in a non-polar solvent such as methane.[48] Lakes composed of a mixture of hydrocarbons, including methane and ethane, have been detected on the surface of Titan by the Cassini spacecraft.
There is debate about the effectiveness of methane and other hydrocarbons as a solvent for life compared to water or ammonia.[54][55][56] Water is a stronger solvent than the hydrocarbons, enabling easier transport of substances in a cell.[57] However, water is also more chemically reactive and can break down large organic molecules through hydrolysis.[54] A life-form whose solvent was a hydrocarbon would not face the threat of its biomolecules being destroyed in this way.[54] Also, the water molecule's tendency to form strong hydrogen bonds can interfere with internal hydrogen bonding in complex organic molecules.[47] Life with a hydrocarbon solvent could make more use of hydrogen bonds within its biomolecules.[54] Moreover, the strength of hydrogen bonds within biomolecules would be appropriate to a low-temperature biochemistry.[54]
Astrobiologist Chris McKay has argued, on thermodynamic grounds, that if life does exist on Titan's surface, using hydrocarbons as a solvent, it is likely also to use the more complex hydrocarbons as an energy source by reacting them with hydrogen, reducing ethane and acetylene to methane.[58] Possible evidence for this form of life on Titan was identified in 2010 by Darrell Strobel of Johns Hopkins University; a greater abundance of molecular hydrogen in the upper atmospheric layers of Titan compared to the lower layers, arguing for a downward diffusion at a rate of roughly 1025 molecules per second and disappearance of hydrogen near Titan's surface. As Strobel noted, his findings were in line with the effects Chris McKay had predicted if methanogenic life-forms were present.[57][58][59] The same year, another study showed low levels of acetylene on Titan's surface, which were interpreted by Chris McKay as consistent with the hypothesis of organisms reducing acetylene to methane.[57] While restating the biological hypothesis, McKay cautioned that other explanations for the hydrogen and acetylene findings are to be considered more likely: the possibilities of yet unidentified physical or chemical processes (e.g. a non-living surface catalyst enabling acetylene to react with hydrogen), or flaws in the current models of material flow.[60] He noted that even a non-biological catalyst effective at 95 K would in itself be a startling discovery.[60]
Azotosome
A hypothetical cell membrane termed an azotosome, capable of functioning in liquid methane in Titan conditions was computer-modeled in an article published in February 2015. Composed of acrylonitrile, a small molecule containing carbon, hydrogen, and nitrogen, it is predicted to have stability and flexibility in liquid methane comparable to that of a phospholipid bilayer (the type of cell membrane possessed by all life on Earth) in liquid water.[61][62] An analysis of data obtained using the Atacama Large Millimeter / submillimeter Array (ALMA), completed in 2017, confirmed substantial amounts of acrylonitrile in Titan's atmosphere.[63][64] Later studies questioned whether acrylonitrile would be able to self-assemble into azotozomes.[65]
Hydrogen fluoride
Hydrogen fluoride (HF), like water, is a polar molecule, and due to its polarity it can dissolve many ionic compounds. At atmospheric pressure, its melting point is 189.15 K (−84.00 °C), and its boiling point is 292.69 K (19.54 °C); the difference between the two is a little more than 100 K. HF also makes hydrogen bonds with its neighbor molecules, as do water and ammonia. It has been considered as a possible solvent for life by scientists such as Peter Sneath[66] and Carl Sagan.[46]
HF is dangerous to the systems of molecules that Earth-life is made of, but certain other organic compounds, such as paraffin waxes, are stable with it.[46] Like water and ammonia, liquid hydrogen fluoride supports an acid–base chemistry. Using a solvent system definition of acidity and basicity, nitric acid functions as a base when it is added to liquid HF.[67]
However, hydrogen fluoride is cosmically rare, unlike water, ammonia, and methane.[68]
Hydrogen sulfide
Hydrogen sulfide is the closest chemical analog to water,[69] but is less polar and is a weaker inorganic solvent.[70] Hydrogen sulfide is quite plentiful on Jupiter's moon Io and may be in liquid form a short distance below the surface; astrobiologist Dirk Schulze-Makuch has suggested it as a possible solvent for life there.[71] On a planet with hydrogen sulfide oceans, the source of the hydrogen sulfide could come from volcanoes, in which case it could be mixed in with a bit of hydrogen fluoride, which could help dissolve minerals. Hydrogen sulfide life might use a mixture of carbon monoxide and carbon dioxide as their carbon source. They might produce and live on sulfur monoxide, which is analogous to oxygen (O2). Hydrogen sulfide, like hydrogen cyanide and ammonia, suffers from the small temperature range where it is liquid, though that, like that of hydrogen cyanide and ammonia, increases with increasing pressure.
Silicon dioxide and silicates
Silicon dioxide, also known as silica and quartz, is very abundant in the universe and has a large temperature range where it is liquid. However, its melting point is 1,600 to 1,725 °C (2,912 to 3,137 °F), so it would be impossible to make organic compounds in that temperature, because all of them would decompose. Silicates are similar to silicon dioxide and some have lower melting points than silica. Feinberg and Shapiro have suggested that molten silicate rock could serve as a liquid medium for organisms with a chemistry based on silicon, oxygen, and other elements such as aluminium.[72]
Other solvents or cosolvents
Other solvents sometimes proposed:
- Supercritical fluids: supercritical carbon dioxide and supercritical hydrogen.[73]
- Simple hydrogen compounds: hydrogen chloride.[74]
- More complex compounds: sulfuric acid,[40] formamide,[41] methanol.[74]
- Very-low-temperature fluids: liquid nitrogen[42] and hydrogen.[42]
- High-temperature liquids: sodium chloride.[75]
Sulfuric acid in liquid form is strongly polar. It remains liquid at higher temperatures than water, its liquid range being 10 °C to 337 °C at a pressure of 1 atm, although above 300 °C it slowly decomposes. Sulfuric acid is known to be abundant in the clouds of Venus, in the form of aerosol droplets. In a biochemistry that used sulfuric acid as a solvent, the alkene group (C=C), with two carbon atoms joined by a double bond, could function analogously to the carbonyl group (C=O) in water-based biochemistry.[40]
A proposal has been made that life on Mars may exist and be using a mixture of water and hydrogen peroxide as its solvent.[76] A 61.2% (by mass) mix of water and hydrogen peroxide has a freezing point of −56.5 °C and tends to super-cool rather than crystallize. It is also hygroscopic, an advantage in a water-scarce environment.[77][78]
Supercritical carbon dioxide has been proposed as a candidate for alternative biochemistry due to its ability to selectively dissolve organic compounds and assist the functioning of enzymes and because "super-Earth"- or "super-Venus"-type planets with dense high-pressure atmospheres may be common.[73]
Other speculations
Non-green photosynthesizers
Physicists have noted that, although photosynthesis on Earth generally involves green plants, a variety of other-colored plants could also support photosynthesis, essential for most life on Earth, and that other colors might be preferred in places that receive a different mix of stellar radiation than Earth.[79][80] These studies indicate that blue plants would be unlikely; however yellow or red plants may be relatively common.[80]
Variable environments
Many Earth plants and animals undergo major biochemical changes during their life cycles as a response to changing environmental conditions, for example, by having a spore or hibernation state that can be sustained for years or even millennia between more active life stages.[81] Thus, it would be biochemically possible to sustain life in environments that are only periodically consistent with life as we know it.
For example, frogs in cold climates can survive for extended periods of time with most of their body water in a frozen state,[81] whereas desert frogs in Australia can become inactive and dehydrate in dry periods, losing up to 75% of their fluids, yet return to life by rapidly rehydrating in wet periods.[82] Either type of frog would appear biochemically inactive (i.e. not living) during dormant periods to anyone lacking a sensitive means of detecting low levels of metabolism.
Alanine world and hypothetical alternatives
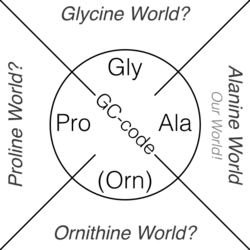
The genetic code may have evolved during the transition from the RNA world to a protein world.[83] The Alanine World Hypothesis postulates that the evolution of the genetic code (the so-called GC phase[84]) started with only four basic amino acids: alanine, glycine, proline and ornithine (now arginine).[85] The evolution of the genetic code ended with 20 proteinogenic amino acids. From a chemical point of view, most of them are Alanine-derivatives particularly suitable for the construction of α-helices and β-sheets – basic secondary structural elements of modern proteins. Direct evidence of this is an experimental procedure in molecular biology known as alanine scanning.
A hypothetical "Proline World" would create a possible alternative life with the genetic code based on the proline chemical scaffold as the protein backbone. Similarly, a "Glycine World" and "Ornithine World" are also conceivable, but nature has chosen none of them.[86] Evolution of life with Proline, Glycine, or Ornithine as the basic structure for protein-like polymers (foldamers) would lead to parallel biological worlds. They would have morphologically radically different body plans and genetics from the living organisms of the known biosphere.[87]
Nonplanetary life
Dusty plasma-based
In 2007, Vadim N. Tsytovich and colleagues proposed that lifelike behaviors could be exhibited by dust particles suspended in a plasma, under conditions that might exist in space.[88][89] Computer models showed that, when the dust became charged, the particles could self-organize into microscopic helical structures, and the authors offer "a rough sketch of a possible model of...helical grain structure reproduction".
Cosmic necklace-based
In 2020, Luis A. Anchordoqu and Eugene M. Chudnovsky of the City University of New York hypothesized that cosmic necklace-based life composed of magnetic monopoles connected by cosmic strings could evolve inside stars.[5] This would be achieved by a stretching of cosmic strings due to the star's intense gravity, thus allowing it to take on more complex forms and potentially form structures similar to the RNA and DNA structures found within carbon-based life. As such, it's theoretically possible that such beings could eventually become intelligent and construct a civilization using the power generated by the star's nuclear fusion. Because such use would use up part of the star's energy output, the luminosity would also fall. For this reason, it's thought that such life might exist inside stars observed to be cooling faster or dimmer than current cosmological models predict.
Life on a neutron star
Frank Drake suggested in 1973 that intelligent life could inhabit neutron stars.[90] Physical models in 1973 implied that Drake's creatures would be microscopic. In 1980, Robert L Forward wrote the science fiction novel Dragon's Egg using Drake's suggestion as a thesis.[91]
Scientists who have published on this topic
Scientists who have considered possible alternatives to carbon-water biochemistry include:
- J. B. S. Haldane (1892–1964), a geneticist noted for his work on abiogenesis.[49]
- V. Axel Firsoff (1910–1981), British astronomer.[92]
- Isaac Asimov (1920–1992), biochemist and science fiction writer.[48]
- Fred Hoyle (1915–2001), astronomer and science fiction writer.
- Norman Horowitz (1915–2005) Caltech geneticist who devised the first experiments carried out to detect life on Mars.[14]
- George C. Pimentel (1922–1989), American chemist, University of California, Berkeley.[93]
- Peter Sneath (1923–2011), microbiologist, author of the book Planets and Life.[66]
- Gerald Feinberg (1933–1992), physicist and Robert Shapiro (1935–2011), chemist, co-authors of the book Life Beyond Earth.[94][95]
- Carl Sagan (1934–1996), astronomer,[93] science popularizer, and SETI proponent.
- Jonathan Lunine (born 1959), American planetary scientist and physicist.
- Robert Freitas (born 1952), specialist in nano-technology and nano-medicine.[96][97]
- John Baross (born 1940), oceanographer and astrobiologist, who chaired a committee of scientists under the United States National Research Council that published a report on life's limiting conditions in 2007.[98][99]
See also
- Abiogenesis
- Astrobiology
- Carbon chauvinism
- Carbon-based life
- Earliest known life forms
- Extraterrestrial life
- Hachimoji DNA
- Iron–sulfur world hypothesis
- Nexus for Exoplanet System Science
- Non-cellular life
- Non-proteinogenic amino acids
- Nucleic acid analogues
- Planetary habitability
- Shadow biosphere
References
- ↑ David J. Des Marais (2008). "The NASA Astrobiology Roadmap". Astrobiology 8 (4): 715–730. doi:10.1089/ast.2008.0819. PMID 18793098. Bibcode: 2008AsBio...8..715D. https://zenodo.org/record/1065569.
- ↑ Davila, Alfonso F.; McKay, Christopher P. (May 27, 2014). "Chance and Necessity in Biochemistry: Implications for the Search for Extraterrestrial Biomarkers in Earth-like Environments". Astrobiology 14 (6): 534–540. doi:10.1089/ast.2014.1150. PMID 24867145. Bibcode: 2014AsBio..14..534D.
- ↑ Singer, Emily (July 19, 2015). "Chemists Invent New Letters for Nature's Genetic Alphabet". Wired. https://www.wired.com/2015/07/chemists-invent-new-letters-natures-genetic-alphabet/. Retrieved July 20, 2015.
- ↑ Kovac, Andreja (1 Apr 2007). "Diazenedicarboxamides as inhibitors of D-alanine-D-alanine ligase (Ddl)". Bioorganic & Medicinal Chemistry Letters 17 (7): 2047–2054. doi:10.1016/j.bmcl.2007.01.015. PMID 17267218. https://www.sciencedirect.com/science/article/pii/S0960894X07000534. Retrieved 12 May 2022.
- ↑ 5.0 5.1 Anchordoqui, Luis A.; Chudnovsky, Eugene M. (2020-08-29). "Can Self-Replicating Species Flourish in the Interior of a Star?" (in en). Letters in High Energy Physics 2020: 166. doi:10.31526/lhep.2020.166. ISSN 2632-2714. Bibcode: 2020LHEP....3..166A. http://journals.andromedapublisher.com/index.php/LHEP/article/view/166.
- ↑ Tsytovich, V. N.; Morfill, G. E.; Fortov, V. E.; Gusein-Zade, N. G.; Klumov, B. A.; Vladimirov, S. V. (2007). "From plasma crystals and helical structures towards inorganic living matter". New Journal of Physics 9 (8): 263. doi:10.1088/1367-2630/9/8/263. Bibcode: 2007NJPh....9..263T.
- ↑ Hellingwerf, Klaas J.; Crielaard, Wim; Westerhoff, Hans V. (1993). "Comparison of Retinal-Based and Chlorophyll-Based Photosynthesis: A Biothermokinetic Description of Photochemical Reaction Centers". Modern Trends in Biothermokinetics. pp. 45–52. doi:10.1007/978-1-4615-2962-0_9. ISBN 978-1-4613-6288-3. https://link.springer.com/chapter/10.1007/978-1-4615-2962-0_9.
- ↑ Davies, P. C. W.; Benner, S.A.; Cleland, C.E.; Lineweaver, C.H.; McKay, C.P.; Wolfe-Simon, F. (2009). "Signatures of a Shadow Biosphere". Astrobiology 9 (2): 241–249. doi:10.1089/ast.2008.0251. PMID 19292603. Bibcode: 2009AsBio...9..241D.
- ↑ Cleland, Carol E.; Copley, Shelley D. (16 January 2006). "The possibility of alternative microbial life on Earth". International Journal of Astrobiology 4 (3–4): 165. doi:10.1017/S147355040500279X. Bibcode: 2005IJAsB...4..165C. archived at [1] (2009-03-20) from original [2]
- ↑ P.C.W. Davies; Charles H. Lineweaver (2005). "Hypothesis Paper: Finding a Second Sample of Life on Earth". Astrobiology 5 (2): 154–63. doi:10.1089/ast.2005.5.154. PMID 15815166. Bibcode: 2005AsBio...5..154D. http://www.mso.anu.edu.au/~charley/papers/DaviesLineweaverOnline05.pdf.
- ↑ Sagan, Carl; Agel, Jerome (2000). Carl Sagan's Cosmic Connection: an Extraterrestrial Perspective (2nd ed.). Cambridge U.P.. p. 41. ISBN 978-0-521-78303-3.
- ↑ 12.0 12.1 Sagan, Carl (2000). Carl Sagan's Cosmic Connection: an Extraterrestrial Perspective (2nd ed.). Cambridge U.P.. p. 46.
- ↑ Sagan, Carl (2000). Carl Sagan's Cosmic Connection: an Extraterrestrial Perspective (2nd ed.). Cambridge U.P.. p. 47.
- ↑ 14.0 14.1 Horowitz, N.H. (1986). Utopia and Back and the search for life in the solar system. New York: W.H. Freeman and Company. ISBN:0-7167-1766-2
- ↑ 15.0 15.1 15.2 Pace, N. R. (2001). "The universal nature of biochemistry". Proceedings of the National Academy of Sciences of the United States of America 98 (3): 805–808. doi:10.1073/pnas.98.3.805. PMID 11158550. Bibcode: 2001PNAS...98..805P.
- ↑ Gillette, Stephen (1996). World-Building. Writer's Digest Books. ISBN 978-0-89879-707-7.
- ↑ Lazio, Joseph. "F.10 Why do we assume that other beings must be based on carbon? Why couldn't organisms be based on other substances?". [sci.astro] ET Life (Astronomy Frequently Asked Questions). http://www.faqs.org/faqs/astronomy/faq/part6/section-16.html.
- ↑ "Astrobiology". Biology Cabinet. September 26, 2006. http://biocab.org/Astrobiology.html.
- ↑ Cairns-Smith, A. Graham (1985). Seven Clues to the Origin of Life. Cambridge: Cambridge University Press. ISBN 978-0-521-27522-4. https://archive.org/details/sevencluestoorig00cair_0.
- ↑ Dawkins, Richard (1996). The Blind Watchmaker. New York: W. W. Norton & Company, Inc. pp. 148–161. ISBN 978-0-393-31570-7. https://archive.org/details/blindwatchmaker0000dawk/page/148.
- ↑ Kan, S. B. Jennifer; Lewis, Russell D.; Chen, Kai; Arnold, Frances H. (2016-11-25). "Directed evolution of cytochrome c for carbon–silicon bond formation: Bringing silicon to life" (in en). Science 354 (6315): 1048–1051. doi:10.1126/science.aah6219. ISSN 0036-8075. PMID 27885032. Bibcode: 2016Sci...354.1048K.
- ↑ William Bains. "Astrobiology—the nature of life". WilliamBains.co.uk. http://www.williambains.co.uk/astrobiology/life2.html.
- ↑ William Bains (June 2004). "Many Chemistries Could Be Used to Build Living Systems". Astrobiology 4 (2): 137–167. doi:10.1089/153110704323175124. PMID 15253836. Bibcode: 2004AsBio...4..137B.
- ↑ 24.0 24.1 "Life-like cells are made of metal". New Scientist. September 14, 2011. https://www.newscientist.com/article/dn20906-lifelike-cells-are-made-of-metal.html. Retrieved 2014-05-25.
- ↑ Early Archaean Microorganisms Preferred Elemental Sulfur, Not Sulfate Science AAAS, by Philippot, et al., (14 September 2007)
- ↑ "Biochemical Periodic Table – Arsenic". UMBBD. 2007-06-08. http://umbbd.ethz.ch/periodic/elements/as.html.
- ↑ Niggemyer, A; Spring S; Stackebrandt E; Rosenzweig RF (December 2001). "Isolation and characterization of a novel As(V)-reducing bacterium: implications for arsenic mobilization and the genus Desulfitobacterium". Appl Environ Microbiol 67 (12): 5568–80. doi:10.1128/AEM.67.12.5568-5580.2001. PMID 11722908. Bibcode: 2001ApEnM..67.5568N.
- ↑ Reilly, Michael (26 April 2008). "Early life could have relied on 'arsenic DNA'". New Scientist 198 (2653): 10. doi:10.1016/S0262-4079(08)61007-6. https://www.newscientist.com/channel/life/mg19826533.600-early-life-could-have-relied-on-arsenic-dna.html.
- ↑ Westheimer, F. H. (1987-03-06). "Why nature chose phosphates". Science 235 (4793): 1173–1178 (see pp. 1175–1176). doi:10.1126/science.2434996. PMID 2434996. Bibcode: 1987Sci...235.1173W. http://academic.evergreen.edu/curricular/m2o2006/seminar/westheimer.pdf. Retrieved 2010-12-03.
- ↑ "NASA-Funded Research Discovers Life Built With Toxic Chemical". NASA.gov. 2 December 2010. http://www.nasa.gov/topics/universe/features/astrobiology_toxic_chemical.html.
- ↑ 31.0 31.1 Wolfe-Simon, Felisa; Blum, Jodi Switzer; Kulp, Thomas R.; Gordon, Shelley E.; Hoeft, S. E.; Pett-Ridge, Jennifer; Stolz, John F.; Webb, Samuel M. et al. (2 December 2010). "A Bacterium That Can Grow by Using Arsenic Instead of Phosphorus". Science 332 (6034): 1163–6. doi:10.1126/science.1197258. PMID 21127214. Bibcode: 2011Sci...332.1163W.
- ↑ Redfield, Rosemary (4 December 2010). "Arsenic-associated bacteria (NASA's claims)". rrresearch.blogspot.com/. http://rrresearch.blogspot.com/2010/12/arsenic-associated-bacteria-nasas.html.
- ↑ Bradley, Alex (5 December 2010). "Arsenate-based DNA: a big idea with big holes". scienceblogs.com/webeasties/. http://scienceblogs.com/webeasties/2010/12/guest_post_arsenate-based_dna.php.
- ↑ Zimmer, Carl (7 December 2010). "Scientists see fatal flaws in the NASA study of arsenic-based life". Slate. http://www.slate.com/id/2276919/.
- ↑ Williams, Sarah (7 November 2012). ""Arsenic Life" Claim Refuted". BioTechniques. http://www.biotechniques.com/news/Arsenic-Life-Claim-Refuted/biotechniques-332691.html#.UQBYhx3O3zk.
- ↑ Basturea GN, Harris TK and Deutscher MP (17 August 2012). "Growth of a bacterium that apparently uses arsenic instead of phosphorus is a consequence of massive ribosome breakdown". J Biol Chem 287 (34): 28816–9. doi:10.1074/jbc.C112.394403. PMID 22798070.
- ↑ Benner, Steven A.; Ricardo, Alonso; Carrigan, Matthew A (2004). "Is there a common chemical model for life in the universe?". Current Opinion in Chemical Biology 8 (6): 676–680. doi:10.1016/j.cbpa.2004.10.003. PMID 15556414.Text as pdf from www.sciencedirect.com (accessed 13 July 2011).
- ↑ Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007; pages 69–79.
- ↑ 39.0 39.1 39.2 39.3 39.4 Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007; p. 72.
- ↑ 40.0 40.1 40.2 Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007; p. 73.
- ↑ 41.0 41.1 41.2 Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007; p. 74.
- ↑ 42.0 42.1 42.2 Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007; p. 75.
- ↑ Benner, Steven A.; Hutter, Daniel (2002-02-01). "Phosphates, DNA, and the Search for Nonterrean Life: A Second Generation Model for Genetic Molecules". Bioorganic Chemistry 30 (1): 62–80. doi:10.1006/bioo.2001.1232. ISSN 0045-2068. https://www.sciencedirect.com/science/article/pii/S0045206801912325.
- ↑ Sagan, Carl (2002). Cosmos. Random House. pp. 126–127. ISBN 978-0-375-50832-5.
- ↑ 45.0 45.1 45.2 Sagan, Carl; Head, Tom (2006). Conversations with Carl Sagan. University Press of Mississippi. p. 10. ISBN 978-1-57806-736-7. https://archive.org/details/conversationswit00saga.
- ↑ 46.0 46.1 46.2 46.3 Sagan, Carl (2002). Cosmos. Random House. p. 128. ISBN 978-0-375-50832-5.
- ↑ 47.0 47.1 47.2 47.3 Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007; page 70.
- ↑ 48.0 48.1 48.2 48.3 48.4 48.5 Isaac Asimov (Winter 1981). "Not as We Know it – the Chemistry of Life". Cosmic Search (North American AstroPhysical Observatory) (9 (Vol 3 No 1)). http://www.bigear.org/CSMO/HTML/CS09/cs09all.htm.
- ↑ 49.0 49.1 J. B. S. Haldane (1954). "The Origins of Life". New Biology 16: 12–27. cited in Darling, David. "Ammonia-based life". http://www.daviddarling.info/encyclopedia/A/ammonialife.html/.
- ↑ 50.0 50.1 50.2 Darling, David. "ammonia-based life". http://www.daviddarling.info/encyclopedia/A/ammonialife.html.
- ↑ Feinberg, Gerald; Robert Shapiro (1980). Life Beyond Earth. Morrow. ISBN 978-0-688-03642-3. https://archive.org/details/lifebeyondearthi0000fein. cited in Darling, David. "ammonia-based life". http://www.daviddarling.info/encyclopedia/A/ammonialife.html/.
- ↑ 52.0 52.1 Schulze-Makuch, Dirk; Irwin, Louis Neal (2008). Life in the Universe: Expectations and Constraints (2 ed.). Springer. p. 119. ISBN 978-3-540-76816-6. https://archive.org/details/lifeuniverseexpe00schu.
- ↑ Fortes, A. D. (1999). "Exobiological Implications of a Possible Ammonia-Water Ocean Inside Titan". http://www.es.ucl.ac.uk/research/planetary/undergraduate/dom/titan/titan.htm.
- ↑ 54.0 54.1 54.2 54.3 54.4 Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007; page 74.
- ↑ McLendon, Christopher; Opalko, F. Jeffrey (March 2015). "Solubility of Polyethers in Hydrocarbons at Low Temperatures. A Model for Potential Genetic Backbones on Warm Titans". Astrobiology 15 (3): 200–206. doi:10.1089/ast.2014.1212. PMID 25761113. Bibcode: 2015AsBio..15..200M.
- ↑ Hadhazy, Adam (13 May 2015). "Alien Life on Oily Exoplanets Could Have Ether-based 'DNA'". Astrobiology Magazine (Space.com). http://www.space.com/29389-alien-life-hydrocarbon-exoplanets-ether-dna.html?adbid=10152808883996466&adbpl=fb&adbpr=17610706465&short_code=2ze6t.
- ↑ 57.0 57.1 57.2 "What is Consuming Hydrogen and Acetylene on Titan?". NASA/JPL. 2010. http://www.jpl.nasa.gov/news/news.cfm?release=2010-190.
- ↑ 58.0 58.1 McKay, C. P.; Smith, H. D. (2005). "Possibilities for methanogenic life in liquid methane on the surface of Titan". Icarus 178 (1): 274–276. doi:10.1016/j.icarus.2005.05.018. Bibcode: 2005Icar..178..274M. https://zenodo.org/record/1259025.
- ↑ Strobel, Darrell F. (2010). "Molecular hydrogen in Titan's atmosphere: Implications of the measured tropospheric and thermospheric mole fractions". Icarus 208 (2): 878–886. doi:10.1016/j.icarus.2010.03.003. Bibcode: 2010Icar..208..878S. http://astrobiology.jhu.edu/wp-content/uploads/2010/06/Icarus-2010-Strobel.pdf.
- ↑ 60.0 60.1 Mckay, Chris (2010). "Have We Discovered Evidence For Life On Titan". New Mexico State University. http://astronomy.nmsu.edu/tharriso/ast105/making_sense.php.html.
- ↑ Stevenson, James; Lunine, Jonathan; Clancy, Paulette (27 Feb 2015). "Membrane alternatives in worlds without oxygen: Creation of an azotosome". Science Advances 1 (1): e1400067. doi:10.1126/sciadv.1400067. PMID 26601130. Bibcode: 2015SciA....1E0067S.
- ↑ Life 'not as we know it' possible on Saturn's moon Titan.
- ↑ Wall, Mike (28 July 2017). "Saturn Moon Titan Has Molecules That Could Help Make Cell Membranes". Space.com. https://www.space.com/37653-saturn-moon-titan-cell-membrane-molecules.html.
- ↑ Palmer, Maureen Y. (28 July 2017). "ALMA detection and astrobiological potential of vinyl cyanide on Titan". Science Advances 3 (7): e1700022. doi:10.1126/sciadv.1700022. PMID 28782019. Bibcode: 2017SciA....3E0022P.
- ↑ Sandström, H.; Rahm, M. (January 2020). "Can polarity-inverted membranes self-assemble on Titan?" (in EN). Science Advances 6 (4): eaax0272. doi:10.1126/sciadv.aax0272. PMID 32042894. Bibcode: 2020SciA....6..272S.
- ↑ 66.0 66.1 Sneath, P. H. A. (1970). Planets and Life. Thames and Hudson. cited in Boyce, Chris (1981). Extraterrestrial Encounter. New English Library. pp. 125, 182.
- ↑ Jander, Gerhart; Spandau, Hans; Addison, C. C. (1971). Chemistry in Nonaqueous Ionizing solvents: Inorganic Chemistry in Liquid Hydrogen Cyanide and Liquid hydrogen Fluoride. II. N.Y.: Pergamon Press. cited in Freitas, Robert A. (1979). "8.2.2". Xenology: An Introduction to the Scientific Study of Extraterrestrial Life, Intelligence, and Civilization. Sacramento, CA: Xenology Research Institute. http://www.xenology.info/Xeno/8.2.2.htm.
- ↑ Freitas, Robert A. (1979). "8.2.2". Xenology: An Introduction to the Scientific Study of Extraterrestrial Life, Intelligence, and Civilization. Sacramento, CA: Xenology Research Institute. http://www.xenology.info/Xeno/8.2.2.htm.
- ↑ Darling, David. "solvent". http://www.daviddarling.info/encyclopedia/S/solvent.html.
- ↑ Jander, J.; Lafrenz, C. (1970). Ionizing Solvents. I. Weinheim/Bergstr.: John Wiley & Sons Ltd., Verlag Chemie. cited in Freitas, Robert A. (1979). "8.2.2". Xenology: An Introduction to the Scientific Study of Extraterrestrial Life, Intelligence, and Civilization. Sacramento, CA: Xenology Research Institute. http://www.xenology.info/Xeno/8.2.2.htm.
- ↑ Choi, Charles Q. (2010-06-10). "The Chance for Life on Io". http://www.astrobio.net/exclusive/3518/the-chance-for-life-on-io.
- ↑ David W. Koerner; Simon LeVay (2000). Here Be Dragons : The Scientific Quest for Extraterrestrial Life. Oxford U.P.. pp. 202. ISBN 978-0-19-803337-0.
- ↑ 73.0 73.1 Budisa, Nediljko; Schulze-Makuch, Dirk (8 August 2014). "Supercritical Carbon Dioxide and Its Potential as a Life-Sustaining Solvent in a Planetary Environment". Life 4 (3): 331–340. doi:10.3390/life4030331. PMID 25370376. Bibcode: 2014Life....4..331B.
- ↑ 74.0 74.1 Ward, Peter D.; Benner, Steven A. (2007). "Alien biochemistries". in Sullivan, Woodruff T.; Baross, John A.. Planets and Life. Cambridge: Cambridge. p. 540. ISBN 978-0-521-53102-3.
- ↑ The methane habitable zone.
- ↑ Houtkooper, Joop M.; Dirk Schulze-Makuch (2007-05-22). "A Possible Biogenic Origin for Hydrogen Peroxide on Mars". International Journal of Astrobiology 6 (2): 147. doi:10.1017/S1473550407003746. Bibcode: 2007IJAsB...6..147H.
- ↑ Houtkooper, Joop M.; Dirk Schulze-Makuch (2007). "The H2O2–H2O Hypothesis: Extremophiles Adapted to Conditions on Mars?". EPSC Abstracts 2: 558. EPSC2007-A-00439. Bibcode: 2007epsc.conf..558H. http://www.cosis.net/abstracts/EPSC2007/00439/EPSC2007-J-00439.pdf.
- ↑ Ellison, Doug (2007-08-24). "Europlanet : Life's a bleach". Planetary.org. http://www.planetary.org/blog/article/00001109/.
- ↑ "NASA – NASA Predicts Non-Green Plants on Other Planets". Nasa.gov. 2008-02-23. http://www.nasa.gov/centers/goddard/news/topstory/2007/spectrum_plants.html.
- ↑ 80.0 80.1 Kiang, Nancy Y.; Segura, Antígona; Tinetti, Giovanna; Jee, Govind; Blankenship, Robert E.; Cohen, Martin; Siefert, Janet; Crisp, David et al. (2007-04-03). "Spectral signatures of photosynthesis. II. Coevolution with other stars and the atmosphere on extrasolar worlds". Astrobiology 7 (1): 252–274. doi:10.1089/ast.2006.0108. PMID 17407410. Bibcode: 2007AsBio...7..252K.
- ↑ 81.0 81.1 "Christmas in Yellowstone". Pbs.org. https://www.pbs.org/wnet/nature/yellowstone/hibernate.html.
- ↑ Main, A. R.; Bentley, P. J. (1964). "Main and Bentley, Ecology, "Water Relations of Australian Burrowing Frogs and Tree Frogs" (1964)". Ecology 45 (2): 379–382. doi:10.2307/1933854.
- ↑ Doig, Abdrew J. (7 December 2016). "Frozen, but no accident – why the 20 standard amino acids were selected". FEBS J. 284 (9): 1296–1305. doi:10.1111/febs.13982. PMID 27926995.
- ↑ Hartman, Hyman; Smith, Temple F. (20 May 2014). "The Evolution of the Ribosome and the Genetic Code". Life 4 (2): 227–249. doi:10.3390/life4020227. PMID 25370196. Bibcode: 2014Life....4..227H.
- ↑ Kubyshkin, Vladimir; Budisa, Nediljko (24 September 2019). "The Alanine World Model for the Development of the Amino Acid Repertoire in Protein Biosynthesis". Int. J. Mol. Sci. 20 (21): 5507. doi:10.3390/ijms20215507. PMID 31694194.
- ↑ Kubyshkin, Vladimir; Budisa, Nediljko (3 July 2019). "Anticipating alien cells with alternative genetic codes: away from the alanine world!". Curr. Opin. Biotechnol. 60: 242–249. doi:10.1016/j.copbio.2019.05.006. PMID 31279217.
- ↑ Budisa, Nediljko; Kubyshkin, Vladimir; Schmidt, Markus (22 April 2020). "Xenobiology: A Journey towards Parallel Life Forms". ChemBioChem 21 (16): 2228–2231. doi:10.1002/cbic.202000141. PMID 32323410.
- ↑ "Physicists Discover Inorganic Dust With Lifelike Qualities". 2007-08-15. https://www.sciencedaily.com/releases/2007/08/070814150630.htm.
- ↑ Tsytovich, V N; G E Morfill, V E Fortov, N G Gusein-Zade, B A Klumov and S V Vladimirov; Fortov, V E; Gusein-Zade, N G; Klumov, B A; Vladimirov, S V (14 August 2007). "From plasma crystals and helical structures towards inorganic living matter". New J. Phys. 9 (263): 263. doi:10.1088/1367-2630/9/8/263. Bibcode: 2007NJPh....9..263T.
- ↑ Drake, F.D. (December 1973). "Life on a Neutron Star: An Interview with Frank Drake". Astronomy: 5–8. https://www.gwern.net/docs/science/1973-drake.pdf.
- ↑ Forward, Robert L (1980). Dragon's Egg. Del REy. pp. 345. ISBN 0-345-28646-4.
- ↑ V. Axel Firsoff (January 1962). "An Ammonia-Based Life". Discovery 23: 36–42. cited in Darling, David. "ammonia-based life". http://www.daviddarling.info/encyclopedia/A/ammonialife.html/.
- ↑ 93.0 93.1 Shklovskii, I.S.; Carl Sagan (1977). Intelligent Life in the Universe. Picador. p. 229.
- ↑ Feinberg, Gerald; Robert Shapiro (1980). Life Beyond Earth. Morrow. ISBN 978-0-688-03642-3. https://archive.org/details/lifebeyondearthi0000fein.
- ↑ A detailed review of this book is: John Gribbin (2 Oct 1980). "Life beyond Earth". New Scientist: xvii.
- ↑ Freitas, Robert A. (1979). Xenology: An Introduction to the Scientific Study of Extraterrestrial Life, Intelligence, and Civilization. Sacramento, CA: Xenology Research Institute. http://www.xenology.info/Xeno.htm.
- ↑ This work is acknowledged the partial basis of the article Darling, David. "ammonia-based life". http://www.daviddarling.info/encyclopedia/A/ammonialife.html/.
- ↑ Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007.
- ↑ Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007; page 5
Further reading
- W. Bains (2004). "Many Chemistries Could Be Used to Build Living Systems". Astrobiology 4 (2): 137–167. doi:10.1089/153110704323175124. PMID 15253836. Bibcode: 2004AsBio...4..137B.
External links
 |