Chemistry:Lanthanum(III) chloride
 Anhydrous
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Other names
Lanthanum trichloride
| |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII |
| ||
| |||
| |||
| Properties | |||
| LaCl3 | |||
| Molar mass | 245.264 g/mol (anhydrous) 353.36 g/mol (hexahydrate) 371.37 g/mol (heptahydrate) | ||
| Appearance | white odorless powder hygroscopic | ||
| Melting point | 858 °C (1,576 °F; 1,131 K) (anhydrous)[1] | ||
| Boiling point | 1,000 °C (1,830 °F; 1,270 K) (anhydrous) | ||
| 957 g/L (25 °C)[1] | |||
| Solubility | soluble in ethanol (heptahydrate) | ||
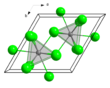
| Structure[2] | |||
| hexagonal (UCl3 type), hP8 | |||
| P63/m, No. 176 | |||
a = 0.74779 nm, b = 0.74779 nm, c = 0.43745 nm
| |||
Formula units (Z)
|
2 | ||
| Tricapped trigonal prismatic,(nine-coordinate) | |||
| Related compounds | |||
Other anions
|
Lanthanum oxide | ||
Other cations
|
Cerium(III) chloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Lanthanum chloride is the inorganic compound with the formula LaCl3. It is a common salt of lanthanum which is mainly used in research. It is a white solid that is highly soluble in water and alcohols.
Preparation
Anhydrous lanthanum(III) chloride can be produced by the ammonium chloride route.[3][4][5] In the first step, lanthanum oxide is heated with ammonium chloride to produce the ammonium salt of the pentachloride:
- La2O3 + 10 NH4Cl → 2 (NH4)2LaCl5 + 6 H2O + 6 NH3
In the second step, the ammonium chloride salt is converted to the trichlorides by heating in a vacuum at 350-400 °C:
- (NH4)2LaCl5 → LaCl3 + 2 HCl + 2 NH3
Uses
Lanthanum chloride is also used in biochemical research to block the activity of divalent cation channels, mainly calcium channels. Doped with cerium, it is used as a scintillator material.[6]
In organic synthesis, lanthanum trichloride functions as a mild Lewis acid for converting aldehydes to acetals.[7]
The compound has been identified as a catalyst for the high pressure oxidative chlorination of methane to chloromethane with hydrochloric acid and oxygen.[8]
Also used in the field of geology as a very dilute solution, which when combined with the proper acids can help identify small >1% Strontium content in powdered rock samples.
References
- ↑ 1.0 1.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedr1 - ↑ Morosin, B (1968). "Crystal Structures of Anhydrous Rare-Earth Chlorides". The Journal of Chemical Physics 49 (7): 3007–3012. doi:10.1063/1.1670543. Bibcode: 1968JChPh..49.3007M.
- ↑ Brauer, G., ed (1963). Handbook of Preparative Inorganic Chemistry (2nd ed.). New York: Academic Press.
- ↑ Meyer, G. (1989). "The Ammonium Chloride Route to Anhydrous Rare Earth Chlorides—The Example of Ycl 3". The Ammonium Chloride Route to Anhydrous Rare Earth Chlorides-The Example of YCl3. Inorganic Syntheses. 25. pp. 146–150. doi:10.1002/9780470132562.ch35. ISBN 978-0-470-13256-2.
- ↑ Edelmann, F. T.; Poremba, P. (1997). Herrmann, W. A.. ed. Synthetic Methods of Organometallic and Inorganic Chemistry. VI. Stuttgart: Georg Thieme Verlag. ISBN 978-3-13-103021-4.
- ↑ Martin, T; Allier, C; Bernard, F (2007). "Lanthanum Chloride Scintillator for X-ray Detection". AIP Conference Proceedings. 879. pp. 1156–1159. doi:10.1063/1.2436269.
- ↑ Lenin, R.; Raju, R. Madhusudhan (2007). "Lanthanum trichloride: An efficient Lewis acid catalyst for chemo and regioselective enamination of β-dicarbonyl compounds". Arkivoc 2007 (13): 204–209. doi:10.3998/ark.5550190.0008.d23.
- ↑ "Methyl chloride production from methane over lanthanum-based catalysts". J. Am. Chem. Soc. 129 (9): 2569–76. 2007. doi:10.1021/ja066913w. PMID 17295483.
 |