Chemistry:AM-630
From HandWiki
Short description: Chemical compound
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
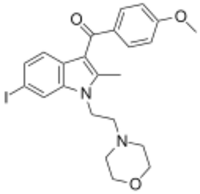
| Formula | C23H25IN2O3 |
| Molar mass | 504.368 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
AM-630 (6-Iodopravadoline) is a drug that acts as a potent and selective inverse agonist for the cannabinoid receptor CB2, with a Ki of 32.1 nM at CB2 and 165x selectivity over CB1, at which it acted as a weak partial agonist.[1][2] It is used in the study of CB2 mediated responses and has been used to investigate the possible role of CB2 receptors in the brain.[3][4] AM-630 is significant as one of the first indole derived cannabinoid ligands substituted on the 6-position of the indole ring, a position that has subsequently been found to be important in determining affinity and efficacy at both the CB1 and CB2 receptors, and has led to the development of many related derivatives.[5][6][7][8][9]
See also
- AM-1221
- Pravadoline
- WIN 54,461 (6-Bromopravadoline)
References
- ↑ "Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630". British Journal of Pharmacology 126 (3): 665–672. February 1999. doi:10.1038/sj.bjp.0702351. PMID 10188977.
- ↑ "The CB2-preferring agonist JWH015 also potently and efficaciously activates CB1 in autaptic hippocampal neurons". Pharmacological Research 66 (5): 437–442. November 2012. doi:10.1016/j.phrs.2012.08.002. PMID 22921769.
- ↑ "Functional CB2 type cannabinoid receptors at CNS synapses". Neuropharmacology 57 (4): 356–368. September 2009. doi:10.1016/j.neuropharm.2009.07.017. PMID 19616018. https://zenodo.org/record/895956.
- ↑ "Brain cannabinoid CB2 receptor in schizophrenia". Biological Psychiatry 67 (10): 974–982. May 2010. doi:10.1016/j.biopsych.2009.09.024. PMID 19931854.
- ↑ "Aminoalkylindoles: structure-activity relationships of novel cannabinoid mimetics". Journal of Medicinal Chemistry 38 (16): 3094–3105. August 1995. doi:10.1021/jm00016a013. PMID 7636873.
- ↑ Hongfeng Deng. Design and synthesis of selective cannabinoid receptor ligands: Aminoalkylindole and other heterocyclic analogs. PhD Dissertation, University of Connecticut, 2000.
- ↑ "C-3 Amido-indole cannabinoid receptor modulators". Bioorganic & Medicinal Chemistry Letters 12 (17): 2399–2402. September 2002. doi:10.1016/S0960-894X(02)00466-3. PMID 12161142.
- ↑ "Indol-3-yl-tetramethylcyclopropyl ketones: effects of indole ring substitution on CB2 cannabinoid receptor activity". Journal of Medicinal Chemistry 51 (6): 1904–1912. March 2008. doi:10.1021/jm7011613. PMID 18311894.
- ↑ Adam, J. M. (2010). "Design, synthesis, and structure–activity relationships of indole-3-carboxamides as novel water soluble cannabinoid CB1 receptor agonists". MedChemComm 1: 54. doi:10.1039/c0md00022a.
 |
