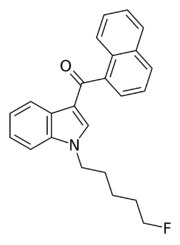
Chemistry:AM-2201
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C24H22FNO |
| Molar mass | 359.444 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
AM-2201 (1-(5-fluoropentyl)-3-(1-naphthoyl)indole) is a recreational designer drug that acts as a potent but nonselective full agonist for the cannabinoid receptor.[2] It is part of the AM series of cannabinoids discovered by Alexandros Makriyannis at Northeastern University.
Hazards
Convulsions have been reported[3] including at doses as low as 10 mg.[4]
Pharmacology
AM-2201 is a full agonist for cannabinoid receptors. Affinities are: with a Ki of 1.0 nM at CB1 and 2.6 nM at CB2.[5] The 4-methyl functional analog MAM-2201 probably has similar affinities.[original research?] AM-2201 has an EC50 of 38 nM for human CB1 receptors, and 58 nM for human CB2 receptors.[6] AM-2201 produces bradycardia and hypothermia in rats at doses of 0.3–3 mg/kg, comparable to the potency of JWH-018 in rats, suggesting potent cannabinoid-like activity.[6]
Pharmacokinetics
AM-2201 metabolism differs only slightly from that of JWH-018. AM-2201 N-dealkylation produces fluoropentane instead of pentane (or plain alkanes in general).[citation needed]
Detection
A forensic standard of AM-2201 is available, and the compound has been posted on the Forendex website of potential drugs of abuse.[7]
Legal status
In the United States, AM-2201 is a Schedule I controlled substance.[8]
See also
References
- ↑ "Substance Details AM-2201". https://www.unodc.org/LSS/Substance/Details/907d3b45-7aa2-41fd-b217-8de909319291.
- ↑ "Bioisosteric Fluorine in the Clandestine Design of Synthetic Cannabinoids". Australian Journal of Chemistry 68 (1): 4–8. 2015. doi:10.1071/CH14198. http://www.publish.csiro.au/?paper=CH14198.
- ↑ "First European case of convulsions related to analytically confirmed use of the synthetic cannabinoid receptor agonist AM-2201". European Journal of Clinical Pharmacology 69 (3): 373–6. March 2013. doi:10.1007/s00228-012-1379-2. PMID 22936123.
- ↑ ekaJ (20 February 2011). "The Night I Killed My Friends". Erowid.org. https://erowid.org/experiences/exp.php?ID=89294.
- ↑ WO patent 0128557, Makriyannis A, Deng H, "Cannabimimetic indole derivatives", granted 2001-06-07
- ↑ 6.0 6.1 "Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135". ACS Chemical Neuroscience 6 (8): 1445–58. August 2015. doi:10.1021/acschemneuro.5b00107. PMID 25921407. https://zenodo.org/record/47750.
- ↑ "Southern Association of Forensic Scientists". http://forendex.southernforensic.org/index.php/detail/index/1097.
- ↑ Controlled Substances listed by the DEA
 |
