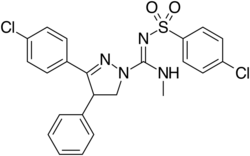
Chemistry:Ibipinabant
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C24H22Cl2N4O2S |
| Molar mass | 501.427 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ibipinabant (SLV319, BMS-646,256) is a drug used in scientific research which acts as a potent and highly selective CB1 antagonist.[1] It has potent anorectic effects in animals,[2] and was researched for the treatment of obesity, although CB1 antagonists as a class have now fallen out of favour as potential anorectics following the problems seen with rimonabant, and so ibipinabant is now only used for laboratory research, especially structure-activity relationship studies into novel CB1 antagonists.[3][4][5] SLV330, which is a structural analogue of Ibipinabant, was reported active in animal models related to the regulation of memory, cognition, as well as in addictive behavior.[6][7] An atom-efficient synthesis of ibipinabant has been reported.[8]
See also
References
- ↑ "Synthesis, biological properties, and molecular modeling investigations of novel 3,4-diarylpyrazolines as potent and selective CB(1) cannabinoid receptor antagonists". Journal of Medicinal Chemistry 47 (3): 627–643. January 2004. doi:10.1021/jm031019q. PMID 14736243.
- ↑ "The relationship of in vivo central CB1 receptor occupancy to changes in cortical monoamine release and feeding elicited by CB1 receptor antagonists in rats". Psychopharmacology 184 (1): 26–35. January 2006. doi:10.1007/s00213-005-0234-x. PMID 16328376.
- ↑ "Novel 3,4-diarylpyrazolines as potent cannabinoid CB1 receptor antagonists with lower lipophilicity". Bioorganic & Medicinal Chemistry Letters 15 (21): 4794–4798. November 2005. doi:10.1016/j.bmcl.2005.07.054. PMID 16140010.
- ↑ "Diaryl dihydropyrazole-3-carboxamides with significant in vivo antiobesity activity related to CB1 receptor antagonism: synthesis, biological evaluation, and molecular modeling in the homology model". Journal of Medicinal Chemistry 50 (24): 5951–5966. November 2007. doi:10.1021/jm061490u. PMID 17979261.
- ↑ "Bioisosteric replacement of dihydropyrazole of 4S-(-)-3-(4-chlorophenyl)-N-methyl-N'-[(4-chlorophenyl)-sulfonyl]-4-phenyl-4,5-dihydro-1H-pyrazole-1-caboxamidine (SLV-319) a potent CB1 receptor antagonist by imidazole and oxazole". Bioorganic & Medicinal Chemistry Letters 18 (3): 963–968. February 2008. doi:10.1016/j.bmcl.2007.12.036. PMID 18207393.
- ↑ "SLV330, a cannabinoid CB1 receptor antagonist, ameliorates deficits in the T-maze, object recognition and Social Recognition Tasks in rodents". Neurobiology of Learning and Memory 93 (4): 522–531. May 2010. doi:10.1016/j.nlm.2010.01.010. PMID 20132903.
- ↑ "SLV330, a cannabinoid CB(1) receptor antagonist, attenuates ethanol and nicotine seeking and improves inhibitory response control in rats". Behavioural Brain Research 217 (2): 408–415. March 2011. doi:10.1016/j.bbr.2010.11.013. PMID 21074574.
- ↑ "An expedient atom-efficient synthesis of the cannabinoid CB1 receptor inverse agonist ibipinabant". Tetrahedron Letters 52 (12): 1303–1305. 2011. doi:10.1016/j.tetlet.2011.01.068.
 |

