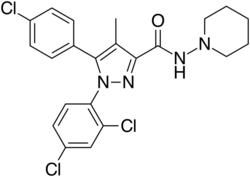
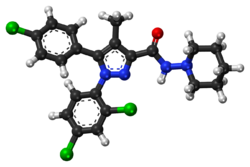
Chemistry:Rimonabant
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Undetermined |
| Protein binding | Nearly 100% |
| Metabolism | Hepatic, CYP3A4 involved |
| Elimination half-life | Variable: 6 to 9 days with normal BMI 16 days if BMI >30 |
| Excretion | Fecal (86%) and renal (3%) |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H21Cl3N4O |
| Molar mass | 463.79 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Rimonabant (also known as SR141716; trade names Acomplia, Zimulti)[3] is an anorectic antiobesity drug approved in Europe in 2006 but was withdrawn worldwide in 2008 due to serious psychiatric side effects; it was never approved in the United States.[1][2] Rimonabant is an inverse agonist for the cannabinoid receptor CB1 and was first-in-class for clinical development.[4][5]
History
Rimonabant is a selective CB1 receptor blocker and was discovered and developed by Sanofi-Aventis.[6]
On 21 June 2006, the European Commission approved the sale of rimonabant in the then-25-member European Union as a prescription drug for use in conjunction with diet and exercise for patients with a body mass index (BMI) greater than 30 kg/m2, or patients with a BMI greater than 27 kg/m2 with associated risk factors, such as type 2 diabetes or dyslipidaemia.[7] It was first in its class to be approved anywhere in the world.[5]
Rimonabant was submitted to the Food and Drug Administration (FDA) for approval in the United States in 2005; in 2007, the FDA's Endocrine and Metabolic Drugs Advisory Committee (EMDAC) concluded that Sanofi-Aventis failed to demonstrate the safety of rimonabant and voted against recommending the anti-obesity treatment for approval.[8] The application was deemed not-approvable by FDA, and the company cancelled plans for a re-submission.[9]
The drug was approved in Brazil in April 2007.[2]
In October 2008, the European Medicines Agency recommended the suspension of Acomplia after the Committee for Medicinal Products for Human Use (CHMP) had determined that the risks of Acomplia outweighed its benefits due to the risk of serious psychiatric problems, including suicide.[10] In November 2008 an advisory committee in Brazil recommended suspension as well, and that month Sanofi-Aventis suspended sale of the drug worldwide.[2] The EMA approval was withdrawn in January 2009.[11][12] In 2009 India prohibited the manufacture and sale of the drug.[13]
Adverse effects
Data from clinical trials submitted to regulatory authorities showed that rimonabant caused depressive disorders or mood alterations in up to 10% of subjects and suicidal ideation in around 1%, and in Europe it was contraindicated for people with any psychiatric disorder, including depressed or suicidal individuals.[7] Data from a large, randomized, clinical trial (CRESCENDO) with > 9000 patients receiving rimonabant treatment demonstrated a rate of psychiatric adverse events (anxiety, depression, depressed mood, or insomnia) of greater than 30%.[14]
Additionally, nausea and upper respiratory tract infections were very common adverse effects (occurring in more than 10% of people); common adverse effects (occurring in between 1% and 10% of people) included gastroenteritis, anxiety, irritability, insomnia and other sleep disorders, hot flushes, diarrhea, vomiting, dry or itchy skin, tendonitis, muscle cramps and spasms, fatigue, flu-like symptoms, and increased risk of falling.
The FDA's advisory committee concurred with concerns raised by the review divisions. Based on human and on animal data, it appeared that the therapeutic window with regard to CNS toxicity, and specifically seizures was narrow.[2][15][16]
EMA postmarketing surveillance data suggested that the risk of psychiatric disorders in people taking rimonabant was doubled.[2]
Pharmacology
Pharmacodynamics
Rimonabant is an inverse agonist of the cannabinoid CB1 receptor. Originally thought to be selective for the CB1 receptor, rimonabant was subsequently also found to act as an antagonist of the μ-opioid receptor.[17]
Chemistry
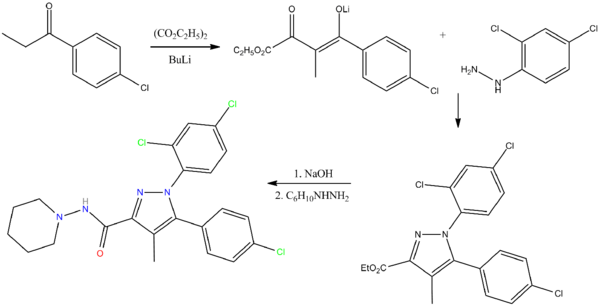
The chemical synthesis of rimonabant is described as follows:[18][failed verification]
Research
Along with the clinical trials in obesity that generated the data submitted to regulatory authorities,[19] rimonabant was also studied in clinical trials[2] for diabetes, atherosclerosis, and smoking cessation.[20][21]
See also
- Drinabant – a cannabinoid receptor antagonist developed for prescription drug use that triggered severe psychiatric side effects.
References
- ↑ 1.0 1.1 "Rimonabant: From RIO to Ban". Journal of Obesity 2011: 432607. 2011. doi:10.1155/2011/432607. PMID 21773005.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "The psychiatric side-effects of rimonabant". Revista Brasileira de Psiquiatria 31 (2): 145–153. June 2009. doi:10.1590/s1516-44462009000200012. PMID 19578688.
- ↑ "Rimonabant" (in en). AdisInsight. http://adisinsight.springer.com/drugs/800007737.
- ↑ "Cannabinoid-1 receptor inverse agonists: current understanding of mechanism of action and unanswered questions". International Journal of Obesity 33 (9): 947–955. September 2009. doi:10.1038/ijo.2009.132. PMID 19597516.
- ↑ 5.0 5.1 "European Approval Comes Early for Sanofi-Aventis' Acomplia". IHS. June 23, 2006. https://www.ihs.com/country-industry-forecasting.html?ID=106599233.
- ↑ "The development of cannabinoid antagonists". Current Medicinal Chemistry 6 (8): 745–755. August 1999. doi:10.2174/0929867306666220401143808. PMID 10469889.
- ↑ 7.0 7.1 "Acomplia EPAR". EMA. January 30, 2009. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000666/WC500021282.pdf. From EMA index page
- ↑ "F.D.A. Panel Rejects Drug for Obesity". The New York Times. 14 June 2007. https://query.nytimes.com/gst/fullpage.html?res=9C0CE7D9143FF937A25755C0A9619C8B63.
- ↑ "Sanofi-Aventis Drops Application for Drug". The New York Times. 30 June 2007. https://www.nytimes.com/2007/06/30/business/30drug.html.
- ↑ "The European Medicines Agency recommends suspension of the marketing authorisation of Acomplia". European Medicines Agency. 23 October 2008. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2009/11/news_detail_000244.jsp.
- ↑ "Anti-obesity drug use suspended". BBC News. 23 October 2008. http://news.bbc.co.uk/2/hi/health/7687311.stm.
- ↑ "Public Statement on Acomplia (rimonabant) Withdrawal of the Marketing Authorisation in the European Union". European Medicines Agency. 30 January 2009. http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2009/11/WC500012189.pdf.
- ↑ "Drugs banned in India". Central Drugs Standard Control Organization, Dte.GHS, Ministry of Health and Family Welfare, Government of India. http://www.cdsco.nic.in/writereaddata/DBC.pdfl.
- ↑ "Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo-controlled trial". Lancet 376 (9740): 517–523. August 2010. doi:10.1016/S0140-6736(10)60935-X. PMID 20709233.
- ↑ "FDA Briefing Document NDA 21-888 Zimulti (rimonabant) Tablets, 20". FDA. June 13, 2007. https://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4306b1-fda-backgrounder.pdf.
- ↑ "Nonclinical Overview: CNS Toxicity with Rimonabant". FDA, Division of Metabolism & Endocrinology Products. June 13, 2007. https://www.fda.gov/ohrms/dockets/ac/07/slides/2007-4306s1-09-FDA-Bruno.ppt.
- ↑ "AM-251 and rimonabant act as direct antagonists at mu-opioid receptors: implications for opioid/cannabinoid interaction studies". Neuropharmacology 63 (5): 905–915. October 2012. doi:10.1016/j.neuropharm.2012.06.046. PMID 22771770.
- ↑ "Studies on hindered phenols and analogues. 1. Hypolipidemic and hypoglycemic agents with ability to inhibit lipid peroxidation". Journal of Medicinal Chemistry 32 (2): 421–428. February 1989. doi:10.1021/jm00122a022. PMID 2913302.
- ↑ "Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial". JAMA 295 (7): 761–775. February 2006. doi:10.1001/jama.295.7.761. PMID 16478899.
- ↑ "Cannabinoid type 1 receptor antagonists for smoking cessation". The Cochrane Database of Systematic Reviews 2011 (3): CD005353. March 2011. doi:10.1002/14651858.CD005353.pub4. PMID 21412887.
- ↑ "Involvement of the endocannabinoid system in drug addiction". Trends in Neurosciences 29 (4): 225–232. April 2006. doi:10.1016/j.tins.2006.01.008. PMID 16483675.
 |