Chemistry:URB597
| |
| Names | |
|---|---|
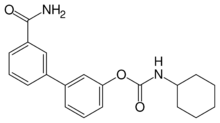
| Preferred IUPAC name
3′-Carbamoyl[1,1′-biphenyl]-3-yl cyclohexylcarbamate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| MeSH | URB597 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C20H22N2O3 | |
| Molar mass | 338.407 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
URB597 (KDS-4103) is a relatively selective and irreversible inhibitor of the enzyme fatty acid amide hydrolase (FAAH).[1][2] FAAH is the primary degradatory enzyme for the endocannabinoid anandamide and, as such, inhibition of FAAH leads to an accumulation of anandamide in the CNS and periphery where it activates cannabinoid receptors. URB597 has been found to elevate anandamide levels and have activity against neuropathic pain in a mouse model.[3]
Preclinical studies have shown FAAH inhibitors to increase BDNF levels in the hippocampus and prefrontal cortex,[4] highlighting their potential in addiction treatment as "enviromimetics".[5] Indeed, Chauvet et al. found that chronic URB597 administration in rats "significantly reduces cocaine-seeking behaviour and cue- and stress-induced relapse".[6]
URB597 was at one point being developed by Kadmus Pharmaceuticals, Inc. for clinical trials in humans.[7]
See also
References
- ↑ Mor, Marco; Rivara, S; Lodola, A; Plazzi, PV; Tarzia, G; Duranti, A; Tontini, A; Piersanti, G et al. (2004). "Cyclohexylcarbamic acid 3'- or 4'-substituted biphenyl-3-yl esters as fatty acid amide hydrolase inhibitors: synthesis, quantitative structure-activity relationships, and molecular modeling studies". J Med Chem 47 (21): 4998–5008. doi:10.1021/jm031140x. PMID 15456244. https://escholarship.org/content/qt19z085c2/qt19z085c2.pdf?t=ovhxc2.
- ↑ Alexander, JP; Cravatt, BF (2005). "Mechanism of Carbamate Inactivation of FAAH: Implications for the Design of Covalent Inhibitors and In Vivo Functional Probes for Enzymes". Chem. Biol. 12 (11): 1179–87. doi:10.1016/j.chembiol.2005.08.011. PMID 16298297.
- ↑ Russo, R; Loverme, J; La Rana, G; Compton, TR; Parrott, J; Duranti, A; Tontini, A; Mor, M et al. (2007). "The fatty-acid amide hydrolase inhibitor URB597 (cyclohexylcarbamicacid 3′-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice". J Pharmacol Exp Ther 322 (1): 236–42. doi:10.1124/jpet.107.119941. PMID 17412883. https://escholarship.org/content/qt6n6471dj/qt6n6471dj.pdf?t=ov4zt9.
- ↑ Bambico, Francis R.; Duranti, Andrea; Nobrega, José N.; Gobbi, Gabriella (March 2016). "The fatty acid amide hydrolase inhibitor URB597 modulates serotonin-dependent emotional behaviour, and serotonin1A and serotonin2A/C activity in the hippocampus". European Neuropsychopharmacology 26 (3): 578–590. doi:10.1016/j.euroneuro.2015.12.027. ISSN 1873-7862. PMID 26747370. https://pubmed.ncbi.nlm.nih.gov/26747370/.
- ↑ Solinas, Marcello; Chauvet, Claudia; Lafay-Chebassier, Claire; Jaafari, Nematollah; Thiriet, Nathalie (2021-02-01). "Environmental enrichment-inspired pharmacological tools for the treatment of addiction" (in en). Current Opinion in Pharmacology 56: 22–28. doi:10.1016/j.coph.2020.09.001. ISSN 1471-4892. PMID 32966941.
- ↑ Chauvet, Claudia; Nicolas, Céline; Thiriet, Nathalie; Lardeux, MD; Virginie; Duranti, Andrea; Solinas, Marcello (2014-12-19). "Chronic Stimulation of the Tone of Endogenous Anandamide Reduces Cue- and Stress-Induced Relapse in Rats". International Journal of Neuropsychopharmacology 18 (1): pyu025. doi:10.1093/ijnp/pyu025. ISSN 1461-1457. PMID 25522382.
- ↑ Kadmus Pharmaceuticals official website
External links
