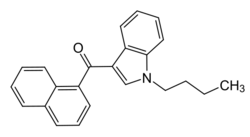
Chemistry:JWH-073
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C23H21NO |
| Molar mass | 327.427 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
JWH-073, a synthetic cannabinoid, is an analgesic chemical from the naphthoylindole family that acts as a full agonist[2] at both the CB1 and CB2 cannabinoid receptors. It is somewhat selective for the CB1 subtype, with affinity at this subtype approximately 5× the affinity at CB2.[3] The abbreviation JWH stands for John W. Huffman, one of the inventors of the compound.
On 20 April 2009, JWH-073 was claimed by researchers at the University of Freiburg to have been found in a "fertiliser" product called "Forest Humus", along with another synthetic cannabinoid (C8)-CP 47,497.[4] These claims were confirmed in July 2009 when tests of Spice product, seized after the legal ban on JWH-018 had gone into effect in Germany, were shown to contain the unregulated compound JWH-073 instead.[5]
Analgesic effects of cannabinoid ligands have been demonstrated in multiple animal pain models (neuropathic, nociceptive).[6]
These compounds work by mimicking the body's naturally-produced endocannabinoid hormones such as 2-arachidonoylglycerol and anandamide, which are biologically active and can exacerbate or inhibit nerve signaling.[6]
As the cause is poorly understood in chronic pain states, more research and development must be done before we can realize the therapeutic potential of this class of biologic compounds.[6]
Pharmacology
JWH-073 has been shown to produce behavioral effects very similar to THC in animals.[7]
Its effects are produced by binding and acting as an agonist to the CB1 and CB2 cannabinoid receptors. The CB1 receptor is found in the brain. JWH-073 binds to CB1 with a higher affinity than THC. CB2 is found outside the brain, mostly in the immune system. The binding with CB2 receptors has been shown to be similar between JWH-073 and THC.[7]
A search in the literature yielded no published studies of the effects of JWH-073 in humans, but these studies in animals suggest with high probability that JWH-073 produces effects very similar to those of THC in humans.[7]
Derivatives
The 4'-methyl derivative of JWH-073 has been encountered as an ingredient of synthetic cannabis blends in Germany and several other European countries since 2010.[8] The 4'-methoxy derivative JWH-080 is also known to be a potent cannabinoid agonist and has been banned in some countries, though it is unclear if it has also been used in synthetic cannabis smoking blends.
Legal status
United States

The US DEA temporarily declared JWH-073 a schedule I controlled substance on 1 March 2011 through 76 FR 11075, and permanently instated the same schedule on 9 July 2012 in the Section 1152 of the Food and Drug Administration Safety and Innovation Act.[9]
Australia
On 8 July 2011 the AUS government banned the sale of JWH-073.[10] JWH-073 is considered a Schedule 9 prohibited substance in Australia under the Poisons Standard (October 2015).[11] A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.[11]
New Zealand
On 8 May 2014 the New Zealand government banned the sale of JWH-073.[12]
Turkey
On 7 January 2011 the Turkey government banned the sale of JWH-073.[13]
See also
References
- ↑ "Grozījumi Ministru kabineta 2005.gada 8.novembra noteikumos Nr.847 "Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem"" (in Latvian). Legal Acts of the Republic of Latvia. http://www.likumi.lv/doc.php?id=201101&from=off.
- ↑ Lisa K. Brents, Anna Gallus-Zawada, Anna Radominska-Pandya, Tamara Vasiljevik, Thomas E Prisinzano, William E Fantegrossi, Jeffery H Moran, Paul L Prather (2012). "Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity". Biochemical Pharmacology 83 (7): 952-961. doi:10.1016/j.bcp.2012.01.004. PMID 22266354. https://pubmed.ncbi.nlm.nih.gov/22266354/. "JWH-073 displays equivalent efficacy to that of the CB1R full agonist CP-55,940".
- ↑ "Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB(1) and CB(2) receptor binding". Drug and Alcohol Dependence 60 (2): 133–40. August 2000. doi:10.1016/S0376-8716(99)00152-0. PMID 10940540.
- ↑ Markuse, Pierre. "Forest Humus – Enthält synthetische Cannabinoide" (in German). Pierre Markuse Blog. http://www.pierre-markuse.de/2009/04/20/forest-humus-enthalt-synthetische-cannabinoide.
- ↑ "Spice: a never ending story?". Forensic Science International 191 (1–3): 58–63. October 2009. doi:10.1016/j.forsciint.2009.06.008. PMID 19589652.
- ↑ 6.0 6.1 6.2 "Dynamic changes to the endocannabinoid system in models of chronic pain". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 367 (1607): 3300–11. December 2012. doi:10.1098/rstb.2011.0390. PMID 23108548.
- ↑ 7.0 7.1 7.2 "DEA Diversion Control Division". http://www.deadiversion.usdoj.gov/drugs_concern/spice/spice_jwh073.html.
- ↑ "EMCDDA Annual Report 2010". http://www.emcdda.europa.eu/attachements.cfm/att_132857_EN_EMCDDA-Europol%20Annual%20Report%202010A.pdf.
- ↑ "Schedules of Controlled Substances: Temporary Placement of Four Synthetic Cannabinoids Into Schedule I". DEA Office of Diversion Control. http://www.deadiversion.usdoj.gov/fed_regs/rules/2014/fr0110_10.htm.
- ↑ "Final Decisions & Reasons for Decisions by Delegates of the Secretary to the Department of Health and Ageing". Department of Health and Ageing. Australian Government. http://www.tga.gov.au/pdf/scheduling/scheduling-decisions-1107-final.pdf.
- ↑ 11.0 11.1 "Poisons Standard". Federal Register of Legislation. Government of Australia. October 2015. https://www.comlaw.gov.au/Details/F2015L01534.
- ↑ "Synthetic cannabis › What they are". NZ Drug Foundation. https://www.drugfoundation.org.nz/synthetic-cannabinoids/what-they-are.
- ↑ › Turkish Drug Law
 |
