Chemistry:AM-1241
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H22IN3O3 |
| Molar mass | 503.340 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
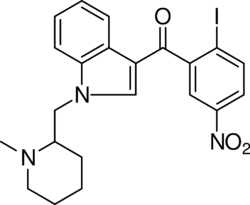
AM-1241 (1-(methylpiperidin-2-ylmethyl)-3-(2-iodo-5-nitrobenzoyl)indole) is a chemical from the aminoalkylindole family that acts as a potent and selective agonist for the cannabinoid receptor CB2,[1][2] with a Ki of 3.4 nM at CB2 and 80 times selectivity over the related CB1 receptor.[3][4] It has analgesic effects in animal studies, particularly against "atypical" pain such as hyperalgesia and allodynia.[5] This is thought to be mediated through CB2-mediated peripheral release of endogenous opioid peptides,[6] as well as direct activation of the TRPA1 channel.[7] It has also shown efficacy in the treatment of amyotrophic lateral sclerosis in animal models.[8][9]
Effects in bone cancer model
The antihyperalgesic effects of AM-1241 were investigated in a murine bone cancer model. Sarcoma cells were injected into the femur of a mouse, and then mice were injected twice daily with AM-1241. Treatment with AM-1241 reduced both spontaneous and evoked pain, as well as reducing the bone loss and subsequent fractures due to the tumor. Pretreatment with the CB2 antagonist SR-144,528 reversed the acute effects of AM-1241 on both spontaneous and evoked pain, while having no effect on its own.[10]
See also
References
- ↑ "In vitro pharmacological characterization of AM1241: a protean agonist at the cannabinoid CB2 receptor?". British Journal of Pharmacology 149 (2): 145–54. September 2006. doi:10.1038/sj.bjp.0706838. PMID 16894349.
- ↑ "Species-specific in vitro pharmacological effects of the cannabinoid receptor 2 (CB2) selective ligand AM1241 and its resolved enantiomers". British Journal of Pharmacology 151 (7): 1061–70. August 2007. doi:10.1038/sj.bjp.0707303. PMID 17549048.
- ↑ "Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS". Proceedings of the National Academy of Sciences of the United States of America 100 (18): 10529–33. September 2003. doi:10.1073/pnas.1834309100. PMID 12917492. Bibcode: 2003PNAS..10010529I.
- ↑ "Recent advances in the development of selective ligands for the cannabinoid CB(2) receptor". Current Topics in Medicinal Chemistry 8 (3): 187–204. 2008. doi:10.2174/156802608783498014. PMID 18289088.
- ↑ "CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms". The European Journal of Neuroscience 23 (6): 1530–8. March 2006. doi:10.1111/j.1460-9568.2006.04684.x. PMID 16553616.
- ↑ "CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids". Proceedings of the National Academy of Sciences of the United States of America 102 (8): 3093–8. February 2005. doi:10.1073/pnas.0409888102. PMID 15705714. Bibcode: 2005PNAS..102.3093I.
- ↑ "Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation". The Journal of Neuroscience 28 (5): 1064–75. January 2008. doi:10.1523/JNEUROSCI.1565-06.2008. PMID 18234885.
- ↑ "AM1241, a cannabinoid CB2 receptor selective compound, delays disease progression in a mouse model of amyotrophic lateral sclerosis". European Journal of Pharmacology 542 (1–3): 100–5. August 2006. doi:10.1016/j.ejphar.2006.05.025. PMID 16781706.
- ↑ "The CB2 cannabinoid agonist AM-1241 prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis when initiated at symptom onset". Journal of Neurochemistry 101 (1): 87–98. April 2007. doi:10.1111/j.1471-4159.2006.04346.x. PMID 17241118.
- ↑ "A cannabinoid 2 receptor agonist attenuates bone cancer-induced pain and bone loss". Life Sciences 86 (17–18): 646–53. April 2010. doi:10.1016/j.lfs.2010.02.014. PMID 20176037.
 |
