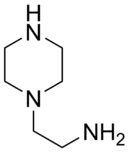
Chemistry:Aminoethylpiperazine
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-(Piperazin-1-yl)ethan-1-amine | |
| Other names
2-(1-Piperazinyl)ethylamine, AEP, N-AEP, N-(2-Aminoethyl)piperazine, 2-Piperazinoethylamine, 1-(2-Aminoethyl)piperazine, 1-Piperazine ethanamine, 1-Aminoethylpiperazine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2815 |
| |
| |
| Properties | |
| C6H15N3 | |
| Molar mass | 129.207 g·mol−1 |
| Appearance | Colourless to yellowish liquid |
| Density | 0.984 g/cm3 at 20 °C |
| Melting point | −19 °C (−2 °F; 254 K) |
| Boiling point | 222 °C (432 °F; 495 K) |
| miscible | |
| Vapor pressure | 0.076 mmHg @ 20 °C |
| Hazards | |
| Main hazards | harmful, corrosive, sensitizing |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H302, H312, H314, H317, H412 | |
| P260, P261, P264, P270, P272, P273, P280, P301+312, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P322, P330, P333+313, P363, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 93 °C (199 °F; 366 K) |
| 315 °C (599 °F; 588 K) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aminoethylpiperazine (AEP) is a derivative of piperazine. This ethyleneamine contains three nitrogen atoms; one primary, one secondary and one tertiary. It is a corrosive organic liquid and can cause second or third degree burns. Aminoethylpiperazine can also cause pulmonary edema as a result of inhalation. It is REACH and TSCA registered.[1]
Production
Ethylene dichloride is reacted with ammonia as a main method of production. This process produces various ethylene amines which can then be purified by distillation. These include ethylenediamine, diethylenetriamine, triethylenetetramine, tetraethylenepentamine, other higher homologues and aminoethyl piperazine. [2][3] AEP is also manufactured by reacting ethylenediamine or ethanolamine/ammonia mixtures over a catalyst.
Epoxy resin curing agent
A key use of AEP is as an epoxy curing agent.[4] When used as an epoxy resin curing agent, it is usually used in conjunction with other amines as an accelerator as it only has 3 amine hydrogens for cross-linking. The tertiary amine on the molecule acts as an accelerator and the other three amine hydrogens allow sites for crosslinking the epoxy.[5] This then allows coating systems to be formulated that prevent corrosion of steel and other substrates.[6] Novolac resins may also be cured by this material and blends.[7]
Other uses
Uses include inhibition of corrosion, surface activation, and as an asphalt additive. As AEP is alkaline and carbon dioxide is weakly acidic, it has been researched as a carbon dioxide sequestrant.[8] This is part of ongoing research in Carbon capture and storage.[9][10]
Toxicology
The toxicology has been extensively studied and is well understood.[11][12]
See also
References
- ↑ PubChem. "1-(2-Aminoethyl)piperazine" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/8795.
- ↑ Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2005. doi:10.1002/14356007.a02_001. ISBN 3527306730.
- ↑ "Epoxide Resins". Plastics Materials (Seventh ed.). Oxford: Butterworth-Heinemann. 1999. pp. 744–777. doi:10.1016/B978-075064132-6/50067-X. ISBN 9780750641326. https://archive.org/details/plasticsmaterial00bryd.
- ↑ Howarth G.A "Synthesis of a legislation compliant corrosion protection coating system based on urethane, oxazolidine and waterborne epoxy technology" Master of Science Thesis April 1997 Imperial College London
- ↑ May, Clayton (2017). Epoxy Resins : Chemistry and Technology, 2nd Edition. London. ISBN 978-1-351-44996-0. OCLC 1004366333. https://www.worldcat.org/oclc/1004366333.
- ↑ Garcia, Filiberto González; Soares, Bluma G.; Pita, Victor J. R. R.; Sánchez, Rubén; Rieumont, Jacques (2007-11-05). "Mechanical properties of epoxy networks based on DGEBA and aliphatic amines" (in en). Journal of Applied Polymer Science 106 (3): 2047–2055. doi:10.1002/app.24895. https://onlinelibrary.wiley.com/doi/10.1002/app.24895.
- ↑ Atta, Ayman M.; Abdou, M. I.; Elsayed, Abdel-Atif A.; Ragab, Mohamed E. (2008-11-01). "New bisphenol novolac epoxy resins for marine primer steel coating applications" (in en). Progress in Organic Coatings 63 (4): 372–376. doi:10.1016/j.porgcoat.2008.06.013. ISSN 0300-9440. https://www.sciencedirect.com/science/article/pii/S0300944008001367.
- ↑ Choi, Jeong Ho; Kim, Young Eun; Nam, Sung Chan; Yun, Soung Hee; Yoon, Yeo Il; Lee, Jung-Hyun (2016-11-01). "CO2 absorption characteristics of a piperazine derivative with primary, secondary, and tertiary amino groups" (in en). Korean Journal of Chemical Engineering 33 (11): 3222–3230. doi:10.1007/s11814-016-0180-9. ISSN 1975-7220. https://doi.org/10.1007/s11814-016-0180-9.
- ↑ Du, Yang; Li, Le; Namjoshi, Omkar; Voice, Alexander K.; Fine, Nathan A.; Rochelle, Gary T. (2013-01-01). "Aqueous Piperazine/N-(2-Aminoethyl) Piperazine for CO2 Capture" (in en). Energy Procedia. GHGT-11 Proceedings of the 11th International Conference on Greenhouse Gas Control Technologies, 18-22 November 2012, Kyoto, Japan 37: 1621–1638. doi:10.1016/j.egypro.2013.06.038. ISSN 1876-6102.
- ↑ Li, Le; Voice, Alexander K.; Li, Han; Namjoshi, Omkar; Nguyen, Thu; Du, Yang; Rochelle, Gary T. (2013). "Amine blends using concentrated piperazine". Energy Procedia 37: 353–369. doi:10.1016/j.egypro.2013.05.121.
- ↑ Leung, Hon-Wing (1994-01-01). "Evaluation of the genotoxic potential of alkyleneamines" (in en). Mutation Research/Genetic Toxicology 320 (1): 31–43. doi:10.1016/0165-1218(94)90057-4. ISSN 0165-1218. PMID 7506385. https://dx.doi.org/10.1016/0165-1218%2894%2990057-4.
- ↑ PubChem. "1-(2-Aminoethyl)piperazine" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/8795.
External links
- Catalytic method for the conjoint manufacture of N-aminoethylpiperazine
- Safety MSDS Data
- Safety data sheet
- Data sheet
 |


