Chemistry:Naphthylpiperazine
From HandWiki
Short description: Chemical compound
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
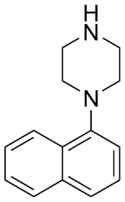
| Formula | C14H16N2 |
| Molar mass | 212.296 g·mol−1 |
| 3D model (JSmol) | |
| |
1-(1-Naphthyl)piperazine (1-NP) is a drug which is a phenylpiperazine derivative. It acts as a non-selective, mixed serotonergic agent, exerting partial agonism at the 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F receptors,[1][2][3] while antagonizing the 5-HT2A, 5-HT2B, and 5-HT2C receptors.[4][5][6] It has also been shown to possess high affinity for the 5-HT3, 5-HT5A, 5-HT6, and 5-HT7 receptors,[7][8][9][10] and may bind to 5-HT4 and the SERT as well.[11][12] In animals it produces effects including hyperphagia,[13][14][15] hyperactivity,[16][17] and anxiolysis,[17][18][19][20] of which are all likely mediated predominantly or fully by blockade of the 5-HT2C receptor.
See also
- Substituted piperazine
- Diphenylpiperazine
- Phenylpiperazine
- Quipazine
References
- ↑ "Interaction of arylpiperazines with 5-HT1A, 5-HT1B, 5-HT1C and 5-HT1D receptors: do discriminatory 5-HT1B receptor ligands exist?". Naunyn-Schmiedeberg's Archives of Pharmacology 339 (6): 675–83. June 1989. doi:10.1007/bf00168661. PMID 2770889.
- ↑ "Molecular cloning and pharmacological characterization of the guinea pig 5-HT1E receptor". European Journal of Pharmacology 484 (2–3): 127–39. January 2004. doi:10.1016/j.ejphar.2003.11.019. PMID 14744596.
- ↑ "Cloning of another human serotonin receptor (5-HT1F): a fifth 5-HT1 receptor subtype coupled to the inhibition of adenylate cyclase". Proceedings of the National Academy of Sciences of the United States of America 90 (2): 408–12. January 1993. doi:10.1073/pnas.90.2.408. PMID 8380639. Bibcode: 1993PNAS...90..408A.
- ↑ "Relative efficacies of piperazines at the phosphoinositide hydrolysis-linked serotonergic (5-HT-2 and 5-HT-1c) receptors". The Journal of Pharmacology and Experimental Therapeutics 242 (2): 552–7. August 1987. PMID 3039120. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=3039120.
- ↑ "Characterization of the serotonin receptor mediating contraction in the mouse thoracic aorta and signal pathway coupling". The Journal of Pharmacology and Experimental Therapeutics 297 (1): 88–95. April 2001. PMID 11259531. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=11259531.
- ↑ "Molecular cloning, functional expression, and mRNA tissue distribution of the human 5-hydroxytryptamine2B receptor". Molecular Pharmacology 46 (2): 227–34. August 1994. PMID 8078486. http://molpharm.aspetjournals.org/cgi/pmidlookup?view=long&pmid=8078486.
- ↑ "Binding of arylpiperazines to 5-HT3 serotonin receptors: results of a structure-affinity study". European Journal of Pharmacology 168 (3): 387–92. September 1989. doi:10.1016/0014-2999(89)90802-9. PMID 2583244.
- ↑ Wesołowska A (2002). "In the search for selective ligands of 5-HT5, 5-HT6 and 5-HT7 serotonin receptors". Polish Journal of Pharmacology 54 (4): 327–41. PMID 12523486.
- ↑ "1-(1-Naphthyl)piperazine as a novel template for 5-HT6 serotonin receptor ligands". Bioorganic & Medicinal Chemistry Letters 15 (6): 1707–11. March 2005. doi:10.1016/j.bmcl.2005.01.031. PMID 15745826.
- ↑ "Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase". The Journal of Biological Chemistry 268 (31): 23422–6. November 1993. doi:10.1016/S0021-9258(19)49479-9. PMID 8226867. http://www.jbc.org/cgi/pmidlookup?view=long&pmid=8226867.
- ↑ "New arylpiperazine derivatives as antagonists of the human cloned 5-HT(4) receptor isoforms". Journal of Medicinal Chemistry 43 (20): 3761–9. October 2000. doi:10.1021/jm0009538. PMID 11020291. https://hal-universite-paris-saclay.archives-ouvertes.fr/hal-03611715/file/Version%20HAL.pdf.
- ↑ "Design and synthesis of long-chain arylpiperazines with mixed affinity for serotonin transporter (SERT) and 5-HT(1A) receptor". The Journal of Pharmacy and Pharmacology 57 (10): 1319–27. October 2005. doi:10.1211/jpp.57.10.0011. PMID 16259761. http://openurl.ingenta.com/content/nlm?genre=article&issn=0022-3573&volume=57&issue=10&spage=1319&aulast=Perrone.
- ↑ "Evidence that hypophagia induced by mCPP and TFMPP requires 5-HT1C and 5-HT1B receptors; hypophagia induced by RU 24969 only requires 5-HT1B receptors". Psychopharmacology 96 (1): 93–100. 1988. doi:10.1007/BF02431539. PMID 2906446.
- ↑ "d-Fenfluramine- and d-norfenfluramine-induced hypophagia: differential mechanisms and involvement of postsynaptic 5-HT receptors". European Journal of Pharmacology 242 (1): 83–90. September 1993. doi:10.1016/0014-2999(93)90013-8. PMID 8223940.
- ↑ "1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane (DOI) exerts an anorexic action that is blocked by 5-HT2 antagonists in rats". Psychopharmacology 94 (3): 342–6. 1988. doi:10.1007/bf00174687. PMID 3128809.
- ↑ "Increased serotonergic functions following administration of 1-(1-naphthyl) piperazine in propranolol injected rats". Pakistan Journal of Pharmaceutical Sciences 19 (3): 194. July 2006. PMID 16935825.
- ↑ 17.0 17.1 Kennett GA (1992). "5-HT1C receptor antagonists have anxiolytic-like actions in the rat social interaction model". Psychopharmacology 107 (2–3): 379–84. doi:10.1007/BF02245165. PMID 1352056.
- ↑ "1-(1-naphthyl)-piperazine, a mixed 5-HT1A and 5-HT2A/2C receptor ligand, elicits an anxiolytic-like effect in the open-field test without changes in 5-HT metabolism". Methods and Findings in Experimental and Clinical Pharmacology 24 (3): 151–7. April 2002. doi:10.1358/mf.2002.24.3.802300. PMID 12087877. http://journals.prous.com/journals/servlet/xmlxsl/pk_journals.xml_summaryn_pr?p_JournalId=6&p_RefId=802300.
- ↑ "Evidence that mCPP-induced anxiety in the plus-maze is mediated by postsynaptic 5-HT2C receptors but not by sympathomimetic effects". Neuropharmacology 33 (3–4): 457–65. 1994. doi:10.1016/0028-3908(94)90076-0. PMID 7984284.
- ↑ "Evidence that 5-HT2c receptor antagonists are anxiolytic in the rat Geller-Seifter model of anxiety". Psychopharmacology 114 (1): 90–6. February 1994. doi:10.1007/BF02245448. PMID 7846211.
 |

