Chemistry:Etoperidone
 | |
| Clinical data | |
|---|---|
| Trade names | Several |
| Other names | ST-1191; McN-A-2673-11 |
| Routes of administration | By mouth |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
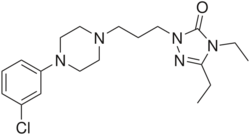
| Formula | C19H28ClN5O |
| Molar mass | 377.92 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Etoperidone, associated with several brand names, is an atypical antidepressant which was developed in the 1970s and either is no longer marketed or was never marketed.[1][2][3] It is a phenylpiperazine related to trazodone and nefazodone in chemical structure and is a serotonin antagonist and reuptake inhibitor (SARI) similarly to them.[4]
Medical uses
Etoperidone was used or was intended for use as an antidepressant in the treatment of depression.[1][5]
Pharmacology
Pharmacodynamics
| Site | Ki (nM) | Species | Ref |
|---|---|---|---|
| NET | 20,000 | Human | [6] |
| DAT | 52,000 | Human | [6] |
| 5-HT1A | 85 | Human | [7] |
| 5-HT2A | 36 | Human | [7] |
| 5-HT2C | ? | ? | ? |
| α1 | 38 | Human | [7] |
| α2 | 570 | Human | [7] |
| D2 | 2,300 | Human | [7] |
| H1 | 3,100 | Human | [7] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | |||
Etoperidone is as an antagonist of several receptors in the following order of potency: 5-HT2A receptor (36 nM) > α1-adrenergic receptor (38 nM) > 5-HT1A receptor (85 nM) (may be a partial agonist) > α2-adrenergic receptor (570 nM);[7] it has only very weak or negligible affinity for blocking the following receptors: D2 receptor (2,300 nM) > H1 receptor (3,100 nM) > mACh receptors (>35,000 nM).[7] In addition to its receptor blockade, etoperidone also has weak affinity for the monoamine transporters as well: serotonin transporter (890 nM) > norepinephrine transporter (20,000 nM) > dopamine transporter (52,000 nM).[6]
Pharmacokinetics
Etoperidone is metabolized in part to meta-chlorophenylpiperazine (mCPP), which likely accounts for its serotonergic effects.[8][9]
Chemistry
Etoperidone is a phenylpiperazine and is chemically related to nefazodone and trazodone.[3][10][11]
History
Etoperidone was discovered by scientists at Angelini, who also discovered trazodone.[12] Its development names have included ST-1191 and McN-A-2673-11.[13][1] The INN etoperidone was proposed in 1976 and recommended in 1977.[14][15] The drug was given brand names in Spain (Centren (Esteve) and Depraser (Lepori)) and Italy (Staff (Sigma Tau))[1] and was also given the brand names Axiomin and Etonin,[13] but it is not entirely clear if it was actually marketed; the Pharmaceutical Manufacturing Encyclopedia provides no dates for commercial introduction.[16] According to Micromedex's Index Nominum: International Drug Directory, etoperidone was indeed previously marketed in Spain and Italy.[1]
Society and culture
Generic names
Etoperidone is the generic name of the drug and its INN, while etoperidone hydrochloride is its USAN.[13][1][5]
Brand names
Etoperidone has been associated with the brand names Axiomin, Centren, Depraser, Etonin, and Staff.[1][13][16]
Research
Etoperidone has been studied in dementia and found to be about as effective as thioridazine.[17]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. p. 421. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA421.
- ↑ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 1533–. ISBN 978-0-8155-1856-3. https://books.google.com/books?id=_J2ti4EkYpkC&pg=PA1533.
- ↑ 3.0 3.1 "Arylpiperazine-Based 5-HT1A Receptor Partial Agonists and 5-HT2A Antagonists for the Treatment of Autism, Depression, Anxiety, Psychosis, and Schizophrenia". Bioactive Heterocyclic Compound Classes: Pharmaceuticals. John Wiley & Sons. 16 August 2012. pp. 81–97. doi:10.1002/9783527664450.ch6. ISBN 978-3-527-66447-4. https://books.google.com/books?id=35uKnWKD9V8C&pg=RA1-PA50.
- ↑ Foundations of Mental Health Care - E-Book. Elsevier Health Sciences. 23 August 2016. pp. 245–. ISBN 978-0-323-37104-9. https://books.google.com/books?id=EFfoDAAAQBAJ&pg=PA245.
- ↑ 5.0 5.1 Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 117–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA117.
- ↑ 6.0 6.1 6.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid9537821 - ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 "Binding of antidepressants to human brain receptors: focus on newer generation compounds". Psychopharmacology 114 (4): 559–65. 1994. doi:10.1007/bf02244985. PMID 7855217.
- ↑ "Active drug metabolites. An overview of their relevance in clinical pharmacokinetics". Clinical Pharmacokinetics 10 (3): 216–227. 1985. doi:10.2165/00003088-198510030-00002. PMID 2861928.
- ↑ "Etoperidone, trazodone and MCPP: in vitro and in vivo identification of serotonin 5-HT1A (antagonistic) activity". Psychopharmacology 108 (3): 320–326. 1992. doi:10.1007/BF02245118. PMID 1387963.
- ↑ "46. Arylalkylamines". Lead optimization for medicinal chemists : pharmacokinetic properties of functional groups and organic compounds. Weinheim: Wiley-VCH. 2012. ISBN 9783527645640.
- ↑ "Atypical Psychotropic Agents" (in en). Drug Discovery and Development. Springer Science & Business Media. 1987. p. 390. ISBN 9781461248286. https://books.google.com/books?id=O0LuBwAAQBAJ&pg=PA390.
- ↑ "Trazodone and the mental pain hypothesis of depression". Neuropsychobiology 15 (Suppl 1): 2–9. 1986. doi:10.1159/000118270. PMID 3014372.
- ↑ 13.0 13.1 13.2 13.3 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 527–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA527.
- ↑ "Proposed INN List 36". Supplement to the WHO Chronicle 30 (9). 1976. https://mednet-communities.net/inn/db/media/docs/p-innlist36.pdf.
- ↑ "Recommended INN List 17". Supplement to the WHO Chronicle 31 (10). 1977. https://mednet-communities.net/inn/db/media/docs/r-innlist17.pdf.
- ↑ 16.0 16.1 Pharmaceutical Manufacturing Encyclopedia. (3rd ed.). Burlington: Elsevier. 2007. p. 1533. ISBN 978-0-8155-1856-3. https://books.google.com/books?id=_J2ti4EkYpkC&pg=PA1533.
- ↑ "Thioridazine for dementia". The Cochrane Database of Systematic Reviews (3): CD000464. 2001. doi:10.1002/14651858.CD000464. PMID 11686961.
External links
 |
