Chemistry:Fluorocyclohexane
From HandWiki
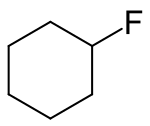
| |
| Names | |
|---|---|
| Preferred IUPAC name
Fluorocyclohexane | |
| Other names
Cyclohexyl fluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H11F | |
| Molar mass | 102.152 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.9280 g/mL |
| Melting point | 13 °C (55 °F; 286 K) |
| Boiling point | 103 °C (217 °F; 376 K) |
| Insoluble | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | DANGER |
| Flash point | 5 °C (41 °F; 278 K) |
| Related compounds | |
Related compounds
|
Chlorocyclohexane Bromocyclohexane Iodocyclohexane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Fluorocyclohexane is an organofluorine compound with the chemical formula (CH
2)
5CHF.[1][2][3]
Synthesis
Fluorocyclohexane is prepared by reaction of cyclohexanol with hydrogen fluoride.[4]
Safety
The compound causes serious skin and eye irritation, and may also cause respiratory irritation.[1]
See also
- Fluoroalkanes
- Fluorocyclopropane
- Fluorobenzene
References
- ↑ 1.0 1.1 "Fluorocyclohexane, 97%, Thermo Scientific Chemicals, Quantity: 5 g | Fisher Scientific". Fisher Scientific. https://www.fishersci.com/shop/products/fluorocyclohexane-97-thermo-scientific-1/AC459320050.
- ↑ "Fluorocyclohexane 372-46-3 | TCI AMERICA". TCI Chemicals. https://www.tcichemicals.com/US/en/p/F0587.
- ↑ (in en) Dictionary of Organic Compounds. CRC Press. 1996. p. 3180. ISBN 978-0-412-54090-5. https://books.google.com/books?id=x2Su3GKCvtsC&dq=Fluorocyclohexane&pg=PA3180. Retrieved 21 June 2023.
- ↑ Olah, George A.; Watkins, Michael (1978). "Fluorinations with Pyridinium Polyhydrogen Fluoride Reagent: 1-Fluoroadamantane". Organic Syntheses 58: 75. doi:10.15227/orgsyn.058.0075.
 |

