Chemistry:Zinc fluoride
| |
| Names | |
|---|---|
| IUPAC name
Zinc(II) fluoride
| |
| Other names
Zinc difluoride
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| UN number | 3077 |
| |
| |
| Properties | |
| ZnF 2 | |
| Molar mass | 103.406 g/mol (anhydrous) 175.45 g/mol (tetrahydrate) |
| Appearance | white needles hygroscopic |
| Density | 4.95 g/cm3 (anhydrous) 2.30 g/cm3 (tetrahydrate) |
| Melting point | 872 °C (1,602 °F; 1,145 K) (anhydrous) 100 °C, decomposes (tetrahydrate) |
| Boiling point | 1,500 °C (2,730 °F; 1,770 K) (anhydrous) |
| .000052 g/(100 mL) (anhydrous) 1.52 g/(100 mL), 20 °C (tetrahydrate) | |
| Solubility | sparingly soluble in HCl, HNO3, ammonia |
| −38.2·10−6 cm3/mol | |
| Structure | |
| tetragonal (anhydrous), tP6 | |
| P42/mnm, No. 136 | |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H301, H315, H318, H335 | |
| P261, P264, P270, P271, P280, P301+310, P302+352, P304+340, P305+351+338, P310, P312, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
|
Other cations
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
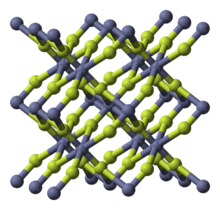
Zinc fluoride is an inorganic chemical compound with the chemical formula ZnF
2. It is encountered as the anhydrous form and also as the tetrahydrate, ZnF
2 · 4H2O (rhombohedral crystal structure).[2] It has a high melting point and has the rutile structure containing 6 coordinate zinc, which suggests appreciable ionic character in its chemical bonding.[3] Unlike the other zinc halides, ZnCl
2, ZnBr
2 and ZnI
2, it is not very soluble in water.[3]
Like some other metal difluorides, ZnF
2 crystallizes in the rutile structure, which features octahedral Zn cations and trigonal planar fluorides.[4]
Preparation and reactions
Zinc fluoride can be synthesized several ways.
- The reaction of zinc metal with fluorine gas.[3]
- Reaction of hydrofluoric acid with zinc, to yield hydrogen gas (H
2) and zinc fluoride (ZnF
2).[3]
Zinc fluoride can be hydrolysed by hot water to form the zinc hydroxide fluoride, Zn(OH)F.[5]
The salt is believed to form both a tetrahydrate and a dihydrate.[6]
References
- ↑ "ZINC fluoride" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/24551#section=Safety-and-Hazards.
- ↑ Perry, D. L.; Phillips, S. L. (1995). Handbook of Inorganic Compounds. CRC Press. ISBN 0-8493-8671-3.
- ↑ 3.0 3.1 3.2 3.3 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Stout, J. W.; Reed, Stanley A. (1954). "The Crystal Structure of MnF2, FeF2, CoF2, NiF2 and ZnF2". J. Am. Chem. Soc. 76 (21): 5279–5281. doi:10.1021/ja01650a005.
- ↑ Srivastava, O. K.; Secco, E. A. (1967). "Studies on Metal Hydroxy Compounds. I. Thermal Analyses of Zinc Derivatives ε-Zn(OH)2, Zn5(OH)8Cl2 · H2O, β-ZnOHCl, and ZnOHF". Canadian Journal of Chemistry 45 (6): 579–583. doi:10.1139/v67-096.
- ↑ Lindahl., Charles B.; Mahmood, Tariq (2000), "Fluorine compounds, inorganic, zinc", Kirk-Othmer Encyclopedia of Chemical Technology, New York: John Wiley, doi:10.1002/0471238961.2609140312091404.a01, ISBN 9780471238966, http://onlinelibrary.wiley.com/book/10.1002/0471238961
External links
- "Zinc Fluoride". American Elements. http://www.americanelements.com/znf.html.
 |


