Chemistry:Rhodium trifluoride
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Rhodium(III) fluoride
| |
| Other names
Rhodium trifluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| RhF3 | |
| Molar mass | 159.90070 g·mol−1 |
| Appearance | red-brown solid |
| Density | 5.38 g/cm3[1] |
| Structure | |
| Trigonal | |
| R3c | |
a = 4.873, c = 13.550[2]
| |
Formula units (Z)
|
6 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rhodium(III) fluoride or rhodium trifluoride is the inorganic compound with the formula RhF3. It is a red-brown, diamagnetic solid.
Synthesis and structure
The compound is prepared by fluorination of rhodium trichloride:
- 2 RhCl
3 + 3 F
2 → 2 RhF
3 + 3 Cl
2
It can also be obtained by direct combination of the elements:[3]
- 2 Rh + 3 F
2 → 2 RhF
3
Anhydrous RhF
3 is insoluble in water and does not react with it, but the hydrates RhF
3 · 6H2O and RhF
3 · 9H2O can be prepared by adding hydrofluoric acid to aqueous rhodium(III) solutions.[3]
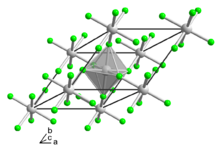
According to X-ray crystallography, the compound adopts the same structure as vanadium trifluoride, wherein the metal achieves octahedral coordination geometry.[2]
References
- ↑ hrsg. von Georg Brauer. Unter Mitarb. von M. Baudler (1975) (in de). Handbuch der präparativen anorganischen Chemie / 1.. Stuttgart: Enke. p. 280. ISBN 3-432-02328-6. OCLC 310719485.
- ↑ 2.0 2.1 L. Grosse, R. Hoppe (1987). "Zur Kenntnis von Sr2RhF7. (Mit einer Bemerkung zur Kristallstruktur von RhF3)". Zeitschrift für Anorganische und Allgemeine Chemie 552 (9): 123–31. doi:10.1002/zaac.19875520914.
- ↑ 3.0 3.1 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 1119-1120. ISBN 978-0-08-037941-8.
 |

