Chemistry:Fenmetozole
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
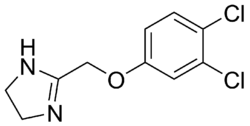
| Formula | C10H10Cl2N2O |
| Molar mass | 245.10 g·mol−1 |
| 3D model (JSmol) | |
| |
Fenmetozole (DH-524) is a drug which was patented as an antidepressant,[1][2] but was later studied as an antagonist of the effects of ethanol, though results were poor and it even increased its effects in some cases.[3][4][5][6][7] It acts as an α2-adrenergic receptor antagonist similarly to other imidazoles like idazoxan.[8] It was never marketed.[2]
Fenoxazoline has the precise same formula, albeit instead of the 3',4'-dich, an ortho-isopropyl group was chosen instead.
References
- ↑ Dictionary of organic compounds. London: Chapman & Hall. 1996. ISBN 0-412-54090-8. https://books.google.com/books?id=rWt8RTCJK1EC&q=fenmetozole&pg=PA3141.
- ↑ 2.0 2.1 David J. Triggle (1997). Dictionary of pharmacological agents. London: Chapman & Hall. ISBN 0-412-46630-9.
- ↑ "Fenmetozole in acute alcohol intoxication in man". Clinical Pharmacology and Therapeutics 17 (6): 735–7. June 1975. doi:10.1002/cpt1975176735. PMID 1095283.
- ↑ "Combined effects of fenmetozole and ethanol". Clinical Pharmacology and Therapeutics 24 (3): 350–3. September 1978. doi:10.1002/cpt1978243350. PMID 357069.
- ↑ "An evaluation of the selectivity of fenmetozole (DH-524) reversal of ethanol-induced changes in central nervous system function". Psychopharmacology 69 (2): 149–55. 1980. doi:10.1007/BF00427641. PMID 6256788.
- ↑ "Differential effects of TRH, amphetamine, naloxone, and fenmetozole on ethanol actions: attenuation of the effects of punishment and impairment of aerial righting reflex". Alcoholism: Clinical and Experimental Research 5 (3): 386–92. 1981. doi:10.1111/j.1530-0277.1981.tb04921.x. PMID 6792942.
- ↑ "An evaluation of the locomotor stimulating action of ethanol in rats and mice". Psychopharmacology 75 (4): 372–9. 1981. doi:10.1007/BF00435856. PMID 6803283.
- ↑ "Effect of methoxy substitution on the adrenergic activity of three structurally related alpha 2-adrenoreceptor antagonists". Journal of Medicinal Chemistry 29 (9): 1780–3. September 1986. doi:10.1021/jm00159a037. PMID 2875186.
 |

