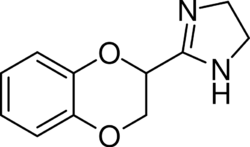
Chemistry:Idazoxan
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C11H12N2O2 |
| Molar mass | 204.229 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Idazoxan (INN) is a drug which is used in scientific research. It acts as both a selective α2 adrenergic receptor antagonist, and an antagonist for the imidazoline receptor.[1][2][3][4] Idazoxan has been under investigation as an antidepressant, but it did not reach the market as such. More recently, it is under investigation as an adjunctive treatment in schizophrenia. Due to its α2 receptor antagonism it is capable of enhancing therapeutic effects of antipsychotics, possibly by enhancing dopamine neurotransmission in the prefrontal cortex of the brain, a brain area thought to be involved in the pathogenesis of schizophrenia.
Alzheimer's research
Mice treated with idazoxan, which blocks the α2A receptor which regulates norepinephrine, behaved similarly to control animals despite still having amyloid-beta plaques in the brain, as a proof-of-concept experiment that dramatically reduced Alzheimer's pathology and symptoms in two mouse models, potentially offering an immediate treatment for this devastating disease.[5]
Synthesis
Note that the literature method claims that the old original patented procedure gives a different reaction product formed through a rearrangement.
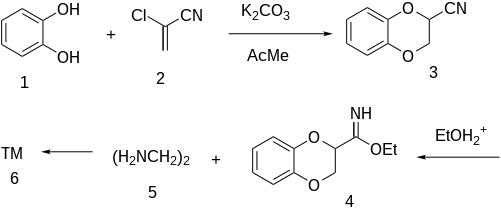
The reaction of catechol (1) with 2-Chloroacrylonitrile [920-37-6] (2) gives 2-cyano-1,4-benzodioxan [1008-92-0] (3). Pinner reaction with alcoholic hydrogen chloride leads to the iminoether,[11] (4). Treatment with ethylenediamine [107-15-3] (5) gives the imidazoline ring affording Idazoxin (6).
See also
References
- ↑ "Participation of imidazoline receptors and alpha(2-)-adrenoceptors in the central hypotensive effects of imidazoline-like drugs". Annals of the New York Academy of Sciences 881 (1): 272–8. June 1999. doi:10.1111/j.1749-6632.1999.tb09369.x. PMID 10415925. Bibcode: 1999NYASA.881..272B.
- ↑ "RX 821002 as a tool for physiological investigation of alpha(2)-adrenoceptors". CNS Drug Reviews 8 (2): 177–92. 2002. doi:10.1111/j.1527-3458.2002.tb00222.x. PMID 12177687.
- ↑ "Idazoxan and brain alpha2-adrenoceptor in the rabbit". Brain Research 436: 289-296. 1988. doi:10.1016/0006-8993(88)90402-7. PMID 2848612.
- ↑ "Imidazole binding sites in rabbit kidney and forebrain membranes". J Auton Pharmacol 11 (4): 277-83. 1991. doi:10.1111/j.1474-8673.1991.tb00325.x. PMID 1939285.
- ↑ "β-amyloid redirects norepinephrine signaling to activate the pathogenic GSK3β/tau cascade". Science Translational Medicine 12 (526). January 2020. doi:10.1126/scitranslmed.aay6931. PMID 31941827.
- ↑ "2-[2-(1, 4-benzodioxanyl)]-2-imidazoline hydrochloride.". Tetrahedron Letters 22 (48): 4839–4842. January 1981. doi:10.1016/S0040-4039(01)92358-5.
- ↑ "An investigation of some base induced transformations of the 1, 4‐benzodioxan ring system.". Journal of Heterocyclic Chemistry 21 (1): 77–80. January 1984. doi:10.1002/jhet.5570210117.
- ↑ "alpha-adrenoreceptor reagents. 1. Synthesis of some 1,4-benzodioxans as selective presynaptic alpha 2-adrenoreceptor antagonists and potential antidepressants". Journal of Medicinal Chemistry 26 (6): 823–31. June 1983. doi:10.1021/jm00360a008. PMID 6133953.
- ↑ Krapcho J, Lott WA, "Certain 1, 4-benzodioxanyl imidazolines and corresponding pyrimidines and process", US patent 2979511, issued 11 April 1961, assigned to Olin Corp.
- ↑ Bougaret J, Avan JL, Segonds R, "Pharmaceutical composition based on idazoxan, salts, hydrates or polymorphs thereof", US patent 7338970, issued 3 March 2008, assigned to Pierre Fabre Medicament.
- ↑ "2,3-Dihydro-1,4-benzodioxin-2-carbimidic acid ethyl ester". PubChem. U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/10035919.
 |
