Chemistry:Pertechnetyl fluoride
From HandWiki
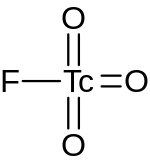
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
| |
| Properties | |
| TcO3F | |
| Molar mass | 165.00 g/mol |
| Appearance | yellow substance |
| Melting point | 18.3 °C (64.9 °F; 291.4 K) |
| Boiling point | 100 °C (212 °F; 373 K) |
| insoluble | |
| Related compounds | |
Related compounds
|
Lanthanum oxyfluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Pertechnetyl fluoride is an inorganic compound, a salt of technetium and hydrofluoric acid with the chemical formula TcO3F. The compound was originally synthesized by H. Selig and G. Malm in 1963.[1][2]
Synthesis
- Effect of fluorine on technetium(IV) oxide at 150 °C:[3]
- 3 TcO
2 + 4 F
2 → 2 TcO
3F + TcF
6
- 3 TcO
- Dissolution of ammonium pertechnetate in anhydrous hydrogen fluoride:
- NH
4TcO
4 + 2 HF → TcO
3F + NH
4F + H
2O
- NH
Physical properties
The compound forms a yellow substance.[4]
Chemical properties
The compound can be hydrolyzed to produce pertechnetic acid and hydrofluoric acid.
- TcO
3F + H
2O → HTcO
4 + HF
It also reacts with arsenic pentafluoride or antimony pentafluoride.[5]
References
- ↑ Schmidbaur, Hubert; Schwarz, W. H. Eugen (21 April 2021). "Permanganyl Fluoride: A Brief History of the Molecule MnO 3 F and of Those Who Cared For It" (in en). Chemistry – A European Journal 27 (23): 6848–6859. doi:10.1002/chem.202004759. ISSN 0947-6539. PMID 33219726.
- ↑ Baran, Enrique J. (1 January 1975). "Vibrational Properties of Pertechnetyl Fluoride". Spectroscopy Letters 8 (8): 599–603. doi:10.1080/00387017508067365. ISSN 0038-7010. Bibcode: 1975SpecL...8..599B. https://www.tandfonline.com/doi/abs/10.1080/00387017508067365. Retrieved 22 March 2023.
- ↑ Selig, H.; Malm, J. G. (1 April 1963). "The preparation and properties of pertechnetyl fluoride, TcO3F" (in en). Journal of Inorganic and Nuclear Chemistry 25 (4): 349–351. doi:10.1016/0022-1902(63)80183-9. ISSN 0022-1902. https://www.sciencedirect.com/science/article/abs/pii/0022190263801839. Retrieved 22 March 2023.
- ↑ Lawroski, Stephen (1963). "Research and development on nonaqueous processing" (in en). Reactor Fuel Processing (U.S. Argonne National Laboratory.) 7 (1): 28. https://books.google.com/books?id=5xzHE8cSNjMC&dq=Pertechnetyl+fluoride&pg=RA11-PA28. Retrieved 22 March 2023.
- ↑ Supeł, Joanna; Abram, Ulrich; Hagenbach, Adelheid; Seppelt, Konrad (1 July 2007). "Technetium Fluoride Trioxide, TcO 3 F, Preparation and Properties" (in en). Inorganic Chemistry 46 (14): 5591–5595. doi:10.1021/ic070333y. ISSN 0020-1669. PMID 17547395. https://pubs.acs.org/doi/10.1021/ic070333y. Retrieved 22 March 2023.
 |
