Chemistry:Potassium tetrafluoronickelate
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| F4K2Ni | |
| Molar mass | 212.8836 g·mol−1 |
| Appearance | green solid |
| Density | 3.36 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
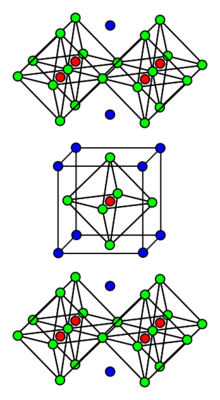
Potassium tetrafluoronickelate is the inorganic compound with the formula K2NiF4. It features octahedral (high spin) Ni centers with Ni-F bond lengths of 2.006 Å. This green solid is a salt of tetrafluoronickelate. It is prepared by melting a mixture of nickel(II) fluoride, potassium fluoride, and potassium bifluoride.[1] The compound adopts a perovskite-like structure consisting of layers of octahedral Ni centers interconnected by doubly bridging fluoride ligands. The layers are interconnected by potassium cations. It is one of the principal Ruddlesden-Popper phases. Early discoveries on cuprate superconductors focused on compounds with structures closely related to K2NiF4, e.g. lanthanum cuprate and derivative lanthanum barium copper oxide.
References
- ↑ Yeh, S. K.; Wu, S. Y.; Lee, C. S.; Wang, Y. (1993). "Electron-Density Distribution in a Crystal of Potassium Tetrafluoronickelate, K2NiF4". Acta Crystallographica Section B: Structural Science 49 (5): 806–811. doi:10.1107/S0108768193003246.
 |


