Chemistry:Tetraethylammonium tetrachloroferrate
From HandWiki
| |

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C8H20Cl4FeN | |
| Molar mass | 327.90 g·mol−1 |
| Appearance | yellow solid |
| Density | 1.358 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
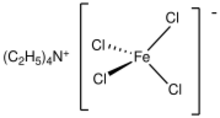
Tetraethylammonium tetrachloroferrate is the chemical compound with the formula (N(C2H5)4)FeCl4.[1] It is the tetraethylammonium salt of the tetrachloroferrate anion, [FeCl4]−.[2]
References
- ↑ Naida S. Gill; F. B. Taylor (1967). "Tetrahalo Complexes of Dipositive Metals in the First Transition Series". Inorganic Syntheses 9: 136–142. doi:10.1002/9780470132401.ch37. ISBN 978-0-470-13240-1.
- ↑ Coucouvanis, D.; Al-Ahmad, Saleem; Kim, C. G.; Mosier, P. E.; Kampf, J. W. (1993). "Oxidative decoupling of the molybdenum-iron-sulfur MoFe3S4 Clusters and possible Relevance to the Oxidative Degradation of the Nitrogenase Cofactor. Isolation and Structural Characterization of the [(Cl4cat)Mo(O)(μ-S)2FeCl2]2- anion". Inorganic Chemistry 32 (9): 1533–1535. doi:10.1021/ic00061a001.
 |

