Earth:Quaternary extinction

The Quaternary period (from 2.588 ± 0.005 million years ago to the present) has seen the extinctions of numerous predominantly megafaunal species, which have resulted in a collapse in faunal density and diversity and the extinction of key ecological strata across the globe. The most prominent event in the Late Pleistocene is differentiated from previous Quaternary pulse extinctions by the widespread absence of ecological succession to replace these extinct species, and the regime shift of previously established faunal relationships and habitats as a consequence.
The earliest casualties were incurred at 130,000 BCE (the start of the Late Pleistocene), in Australia ~ 60,000 years ago, in Americas ~ 15 000 years ago, coinciding in time with the early human migrations.[1][2] However, the great majority of extinctions in Afro-Eurasia and the Americas occurred during the transition from the Pleistocene to the Holocene epoch (13,000 BCE to 8,000 BCE). This extinction wave did not stop at the end of the Pleistocene, continuing, especially on isolated islands, in human-caused extinctions, although there is debate as to whether these should be considered separate events or part of the same event.[3]
Among the main causes hypothesized by paleontologists are overkill by the widespread appearance of humans and natural climate change.[4] A notable modern human presence first appeared during the Middle Pleistocene in Africa,[5] and started to establish continuous, permanent populations in Eurasia and Australasia from 100,000 BCE[6] and 63,000 BCE[7] respectively, and the Americas from 22,000 BCE.[8][9][10][11]
A variant of the former possibility is the second-order predation hypothesis, which focuses more on the indirect damage caused by overcompetition with nonhuman predators. Recent studies have tended to favor the human-overkill theory.[12][13][14][2][15]
Extinctions by biogeographic realm
Summary
| Biogeographic realm | Giants (over 1,000 kg) |
Very large (400–1,000 kg) |
Large (150–400 kg) |
Moderately large (50–150 kg) |
Medium (10–50 kg) |
Total | Regions included | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | Loss | % | Start | Loss | % | Start | Loss | % | Start | Loss | % | Start | Loss | % | start | loss | % | ||
| Afrotropic | 6 | -1 | 16.6% | 4 | -1 | 25% | 25 | -3 | 12% | 32 | -0 | 0% | 69 | -2 | 2.9% | 136 | -7 | 5.1% | Trans-Saharan Africa and Arabia |
| Indomalaya | 5 | -2 | 40% | 6 | -1 | 16.7% | 10 | -1 | 10% | 20 | -3 | 15% | 56 | -1 | 1.8% | 97 | -8 | 8.2% | Indian subcontinent, Southeast Asia, and southern China |
| Palearctic | 8 | -8 | 100% | 10 | -5 | 50% | 14 | -5 | 13.7% | 23 | -3 | 15% | 41 | -1 | 2.4% | 96 | -22 | 22.9% | Eurasia and North Africa |
| Nearctic | 5 | -5 | 100% | 10 | -8 | 80% | 26 | -22 | 84.6% | 20 | -13 | 65% | 25 | -9 | 36% | 86 | -57 | 66% | North America |
| Neotropic | 9 | -9 | 100% | 12 | -12 | 100% | 17 | -14 | 82% | 20 | -11 | 55% | 35 | -5 | 14.3% | 93 | -51 | 54% | South America, Central America, and the Caribbean |
| Australasia | 4 | -4 | 100% | 5 | -5 | 100% | 6 | -6 | 100% | 16 | -13 | 81.2% | 25 | -10 | 40% | 56 | -38 | 67% | Australia , New Guinea, New Zealand, and neighbouring islands. |
| Global | 33 | -26 | 78.8% | 46 | -31 | 67.4% | 86 | -47 | 54.7% | 113 | -41 | 36.3% | 215 | -23 | 10.1% | 493 | -168 | 34% | |
Introduction
The Late Pleistocene saw the extinction of many mammals weighing more than 40 kg. The proportion of megafauna extinctions is progressively larger the further the human migratory distance from Africa, with the highest extinction rates in Australia, and North and South America.
Extinctions in the Americas eliminated all mammals larger than 100 kg of South American origin, including those which migrated north in the Great American Interchange. It was only in Australia and the Americas that extinction occurred at family taxonomic levels or higher. This may relate to non-African megafauna and Homo sapiens not having evolved as species alongside each other. These continents had no known native species of Hominoidea (apes) at all, so no species of Hominidae (greater apes) or Homo.
The increased extent of extinction mirrors the migration pattern of modern humans: the further away from Africa, the more recently humans inhabited the area, the less time those environments (including its megafauna) had to become accustomed to humans (and vice versa).
There is no evidence of megafaunal extinctions at the height of the Last Glacial Maximum, suggesting that increased cold and glaciation were not factors in the Pleistocene extinction.[17]
There are three main hypotheses to explain this extinction:
- climate change associated with the advance and retreat of major ice caps or ice sheets.
- "prehistoric overkill hypothesis"[18]
- the extinction of the woolly mammoth allowed the extensive grassland to become birch forest, then subsequent forest fires changed the climate.[19]
There are some inconsistencies between the current available data and the prehistoric overkill hypothesis. For instance, there are ambiguities around the timing of sudden Australian megafauna extinctions.[18] Evidence supporting the prehistoric overkill hypothesis includes the persistence of megafauna on some islands for millennia past the disappearance of their continental cousins. For instance, Ground sloths survived on the Antilles long after North and South American ground sloths were extinct, woolly mammoths died out on remote Wrangel Island 1,000 years after their extinction on the mainland, while Steller's sea cows persisted off the isolated and uninhabited Commander Islands for thousands of years after they had vanished from the continental shores of the north Pacific.[20] The later disappearance of these island species correlates with the later colonization of these islands by humans.
Alternative hypotheses to the theory of human responsibility include climate change associated with the last glacial period, and the Younger Dryas impact hypothesis as well as Tollmann's hypothesis that extinctions resulted from bolide impacts.
Recent research indicates that each species responded differently to environmental changes, and no one factor by itself explains the large variety of extinctions. The causes may involve the interplay of climate change, competition between species, unstable population dynamics, and human predation.[21]
Afrotropic and Indomalaya: Africa and southern Asia





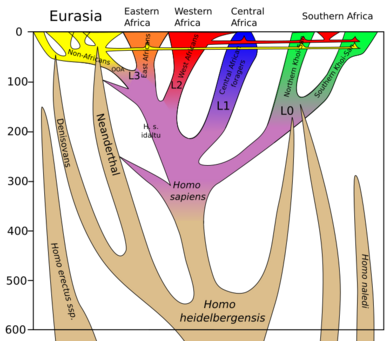
The Afrotropic and Indomalaya biogeographic realms, or Old World tropics, were relatively spared by the Late Pleistocene extinctions. Sub-Saharan Africa and southern Asia are the only regions that have terrestrial mammals weighing over 1000 kg today. However, there are indications of megafaunal extinction events throughout the Pleistocene, particularly in Africa two million years ago, which coincide with key stages of human evolution and climatic trends.[22][23][24] The center of human evolution and expansion, Africa and Asia were inhabited by advanced hominids by 2mya, with Homo habilis in Africa, and Homo erectus on both continents. By the advent and proliferation of Homo sapiens circa 315,000 BCE,[25][26][27] dominant species included Homo heidelbergensis in Africa, the denisovans and neanderthals (fellow H. heidelbergensis descendants) in Eurasia, and Homo erectus in Eastern Asia. Ultimately, on both continents, these groups and other populations of Homo were subsumed by successive radiations of H. sapiens.[28][29][30][31][32][33][34][35] There is evidence of an early migration event 268,000 BCE and later within neanderthal genetics,[36][37][38] however the earliest dating for H. sapiens inhabitation is 118,000 BCE in Arabia, China and Israel,[6][39][40][41] and 71,000 BCE in Indonesia.[42][43] Additionally, not only have these early Asian migrations left a genetic mark on modern Papuan populations,[44][45][46] the oldest known pottery in existence was found in China, dated to 18,000 BCE.[47] Particularly during the late Pleistocene, megafaunal diversity was notably reduced from both these continents, often without being replaced by comparable successor fauna. Climate change has been explored as a prominent cause of extinctions in Southeast Asia.[48]
- Several Bovidae spp.
- Indian aurochs (Bos (primigenius) namadicus) (ancestor to the domestic zebu cattle)
- Bos palaesondaicus (ancestor to the banteng)
- Bison hanaizumiensis[49][50][51]
- Cebu tamaraw (Bubalus cebuensis)
- Bubalus grovesi[52]
- Bubalus wansijocki
- Bubalus mephistopheles
- Giant long-horned buffalo (Pelorovis)
- Megalovis[53]
- Giant hartebeest (Megalotragus)
- Hippotragus spp.
- Rusingoryx
- Sivacobus sankaliai
- Various Gazella spp.[55]
- Sinomegaceros[56]
- Megaceroides algericus
- Sivatherium maurusium
- Dorcabune[57]
- Hippopotamus spp.
- Hippopotamus (Hippopotamus amphibius; extirpated in western Asia circa 1,000 BCE)[58][59]
- All Malagasy hippopotamus spp.
- Hexaprotodon[60]
- Wild Equus spp.
- Several Rhinoceros (Rhinocerotidae) spp.
- Ceratotherium mauritanicum
- Rhinoceros philippinensis[62]
- Rhinoceros sinensis
- South Asian rhinoceros (Rhinoceros sivalensis)
- Giant tapir (Tapirus augustus)
- Various Felidae spp.
- Sivapanthera
- Sri Lanka lion (Panthera leo sinhaleyus)
- Leopard (Panthera pardus; extirpated from Japan [63] and Sumatra)
- Tiger (Panthera tigris; extirpated from Japan ,[64][65] Western and Central Asia, Java, Bali, Borneo and Palawan)
- Ailuropoda baconi (ancestor to the giant panda)
- Aardvark (Orycteropus afer; extirpated in South Asia circa 13,000 BCE)[66][67]
- Stegodon
- Elephas spp.
- Elephas hysudricus
- Elephas hysudrindicus
- Asian Elephant (extirpated in Java and Syria)
- Palaeoloxodon spp.
- Asian straight-tusked elephant (largest land mammal on record)[68]
- Naumann's elephant
- Palaeoloxodon recki
- Paleoloxodon turkmenicus[69]
- Loxodonta atlantica (possible ancestor of the African bush elephant)
- Coryphomys
- Verhoeven's giant tree rat (Papagomys theodorverhoeveni)
- Asian ostrich (Struthio asiaticus)
- Japanese flightless duck (Shiriyanetta hasegawai)[70]
- Bennu heron (Ardea bennuides)
- Leptoptilos robustus
- Megalochelys atlas (largest recorded giant tortoise in existence)
- Hipposideros besaoka
- Giant fossa (Cryptoprocta spelea)
- Microgale macpheei
- Bibymalagasy (aardvark-like mammals endemic to Madagascar)
- Giant members of lemur (Lemuroidea)
- Giant aye-aye (Daubentonia robusta)
- Pachylemur
- Koala lemur (Megaladapis)
- All monkey lemur (Archaeolemuridae) spp.
- All sloth lemur (Palaeopropithecidae) spp.
- Archaeoindris (largest lemur on record)
- Palaeopropithecus
- Babakotia
- Mesopropithecus
- All members of elephant bird, also known as vorompatra in Malagasy language (Aepyornithidae)
- Aepyornis
- Mullerornis
- Vorombe titan (largest bird on record)[71]
- Malagasy sheldgoose (Centrornis)
- Malagasy shelduck (Alopochen sirabensis)
- Hova gallinule (Hovacrex roberti)
- Malagasy lapwing (Vanellus madagascariensis)
- Malagasy crowned eagle (Stephanoaetus maher)
- Ampoza ground roller (Brachypteracias langrandi)
- Voay
- Various Aldabrachelys giant tortoise
- Aldabrachampsus
- Canariomys
- Canary Islands quail (Coturnix gomerae)
- Long-legged bunting (Emberiza alcoveri)
- Centrochelys
- Gallotia goliath
- Several monkey (Simiiformes) spp.
- Macaca spp.
- Macaca anderssoni
- Macaca jiangchuanensis
- Robust macaque (Macaca robustus)
- Gorgopithecus
- Vietnamese Orugutan
- Various Homo spp.
- Archaic African hominins (undescribed)[72]
- Homo erectus
- Flores Man
- Homo luzonensis
- Denisovans (Homo sp.)
- Neanderthals (Homo (sapiens) neanderthalensis)
- Red Deer Cave people (Homo sp.)
- Unknown Asiatic hominins (Homo sp.)[73][74][75]
- Balangoda Man (Homo sapiens balangodensis)
- Macaca spp.
Palearctic: Europe and northern Asia







The Palearctic realm spans the entirety of the European continent and stretches into northern Asia, through the Caucasus and central Asia to northern China, Siberia and Beringia. During the Late Pleistocene, this region was noted for its great diversity and dynamism of biomes, including the warm climes of the Mediterranean basin, open temperate woodlands, arid plains, mountainous heathland and swampy wetlands, all of which were vulnerable to the severe climatic fluctuations of the interchanges between glacial and interglacials periods (stadials). However, it was the expansive mammoth steppe which was the ecosystem which united and defined this region during the Late Pleistocene.[76] One of the key features of Europe's Late Pleistocene climate was the often drastic turnover of conditions and biota between the numerous stadials, which could set within a century. For example, during glacial periods, the entire North Sea was drained of water to form Doggerland. The final major cold spell occurred from 25,000 BCE to 18,000 BCE and is known as the Last Glacial Maximum, when the Fenno-Scandinavian ice sheet covered much of northern Europe, while the Alpine ice sheet occupied significant parts of central-southern Europe.
Europe and northern Asia, being far colder and drier than today,[77] was largely hegemonized by the mammoth steppe, an ecosystem dominated by palatable high-productivity grasses, herbs and willow shrubs.[77][78] This supported an extensive biota of grassland fauna and stretched eastwards from Spain in the Iberian Peninsula to Yukon in modern-day Canada .[76][77][79][80] The area was populated by many species of grazers which assembled in large herds similar in size to those in Africa today. Populous species which roamed the great grasslands included the woolly mammoth, woolly rhinoceros, Elasmotherium, steppe bison, Pleistocene horse, muskox, Cervalces, reindeer, various antelopes (goat-horned antelope, mongolian gazelle, saiga antelope and twisted-horned antelope) and steppe pika. Carnivores included Eurasian cave lion, scimitar cat, cave hyena, grey wolf, dhole and the Arctic fox.[81][82][83]
At the edges of these large stretches of grassland could be found more shrub-like terrain and dry conifer forest and woodland (akin to forest steppe or taiga). The browsing collective of megafauna included woolly rhinoceros, giant deer, moose, Cervalces, tarpan, aurochs, woodland bison, camels and smaller deer (Siberian roe deer, red deer and Siberian musk deer). Brown bears, wolverines, cave bear, wolves, lynx, leopards and red foxes also inhabited this biome. Tigers were at stages also present, from the edges of eastern Europe around the Black Sea to Beringia. The more mountainous terrain, incorporating montane grassland, subalpine conifer forest, alpine tundra and broken, craggy slopes, was occupied by several species of mountain-going animals like argali, chamois, ibex, mouflon, pika, wolves, leopards, Ursus spp. and lynx, with snow leopards, Baikal yak and snow sheep in northern Asia. Arctic tundra, which lined the north of the mammoth steppe, reflected modern ecology with species such as the polar bear, wolf, reindeer and muskox.
Other biomes, although less noted, were significant in contributing to the diversity of fauna in Late Pleistocene Europe. Warmer grasslands such as temperate steppe and Mediterranean savannah hosted Stephanorhinus, gazelle, European bison, Asian ostriches, Leptobos, cheetah and onager. These biomes also contained an assortment of mammoth steppe fauna, such as saiga antelope, lions, scimitar cats, cave hyenas, wolves, Pleistocene horse, steppe bison, twisted-horned antelope, aurochs and camels. Temperate coniferous, deciduous, mixed broadleaf and Mediterranean forest and open woodland accommodated straight-tusked elephants, Praemegaceros, Stephanorhinus, wild boar, bovids such as European bison, tahr and tur, species of Ursus such as the Etruscan bear and smaller deer (Roe deer, red deer, fallow deer and Mediterranean deer) with several mammoth steppe species such as lynx, tarpan, wolves, dholes, moose, giant deer, woodland bison, leopards and aurochs. Woolly rhinoceros and mammoth occasionally resided in these temperate biomes, mixing with predominately temperate fauna to escape harsh glacials.[84][85] In warmer wetlands, European water buffalo and hippopotamus were present. Although these habitats were restricted to micro refugia and to southern Europe and its fringes, being in Iberia, Italy, the Balkans, Ukraine's Black Sea basin, the Caucasus and western Asia, during inter-glacials these biomes had a far more northernly range. For example, hippopotamus inhabited Great Britain and straight-tusked elephant the Netherlands, as recently as 80,000 BCE and 42,000 BCE respectively.[86][87]
The first possible indications of habitation by hominins are the 7.2 million year old finds of Graecopithecus,[88] and 5.7 million year old footprints in Crete — however established habitation is noted in Georgia from 1.8 million years ago, proceeded to Germany and France, by Homo erectus.[89][90] Prominent co-current and subsequent species include Homo antecessor, Homo cepranensis, Homo heidelbergensis, neanderthals and denisovans,[91] preceding habitation by Homo sapiens circa 38,000 BCE. Extensive contact between African and Eurasian Homo groups is known at least in part through transfers of stone-tool technology in 500,000 BCE and again at 250,000 BCE.[72]
Europe's Late Pleistocene biota went through two phases of extinction. Some fauna became extinct before 13,000 BCE, in staggered intervals, particularly between 50,000 BCE and 30,000 BCE. Species include cave bear, Elasmotherium, straight-tusked elephant, Stephanorhinus, water buffalo, neanderthals, gazelle and scimitar cat. However, the great majority of species were extinguished, extirpated or experienced severe population contractions between 13,000 BCE and 9,000 BCE,[92] ending with the Younger Dryas. At that time there were small ice sheets in Scotland and Scandinavia. The mammoth steppe disappeared from the vast majority of its former range, either due to a permanent shift in climatic conditions, or an absence of ecosystem management due to decimated, fragmented or extinct populations of megaherbivores.[93][94] This led to a region wide extinction vortex, resulting in cyclically diminishing bio-productivity[citation needed] and defaunation. Insular species on Mediterranean islands such as Sardinia, Sicily, Malta, Cyprus and Crete, went extinct around the same time as humans colonised those islands. Fauna included dwarf elephants, megacerines and hippopotamuses, and giant avians, otters and rodents.
- Various Bovidae spp.
- Steppe bison (Bison priscus)
- Pleistocene woodland bison (Bison (bonasus) schoetensacki)[95][96]
- Baikal yak (Bos baikalensis)[81]
- European water buffalo (Bubalus murrensis)
- Leptobos spp.
- European tahr (Hemitragus cedrensis)[97][98]
- Giant muskox (Praeovibos priscus)[99]
- Balearic Islands cave goat (Myotragus balearicus)
- Northern saiga antelope (Saiga borealis)[100]
- Twisted-horned antelope (Spirocerus kiakhtensis)[101][82]
- Goat-horned antelope (Parabubalis capricornis)[101][82]
- Gazella spp.[55]
- Various deer (Cervidae) spp.
- Broad-fronted moose (Cervalces latifrons)
- Giant deer (Megaloceros giganteus)
- Praemegaceros
- Cretan dwarf megacerine (Candiacervus)
- Mediterranean deer (Haploidoceros mediterraneus)[102][103]
- Palmated red deer (Cervus elaphus acoronatus)[104]
- All native Hippopotamus spp.[105]
- European hippopotamus (Hippopotamus antiquus)
- Maltese dwarf hippopotamus (Hippopotamus melitensis)
- Cyprus dwarf hippopotamus (Hippopotamus minor)
- Sicilian dwarf hippopotamus (Hippopotamus pentlandi)
- Camelus knoblochi[106] and other Camelus spp.
- Various Equus spp. e.g.
- Wild horse (Equus ferus ssp.)
- Equus cf. gallicus[107][108]
- European Ass (Equus hydruntinus)
- Equus cf. latipes[82][107][109]
- Equus cf. lenensis[82][110]
- Equus cf. uralensis[107]
- All native Rhinoceros (Rhinocerotidae) spp.
- Elasmotherium
- Woolly rhinoceros (Coelodonta antiquitatis)
- Stephanorhinus spp.
- Merck's rhinoceros (Stephanorhinus kirchbergensis)
- Narrow-nosed rhinoceros (Stephanorhinus hemiotoechus)
- Cave wolf (Canis lupus spelaeus)
- Dire wolf (Aenocyon dirus)[111]
- Various Felidae spp.
- Cave hyena (Crocuta crocuta spelaea)
- European dhole (Cuon alpinus europaeus)
- Sardinian dhole (Cynotherium sardous)
- Several otter (Lutrinae) spp.
- Robust Pleistocene European otter (Cyrnaonyx)
- Pleistocene Mediterranean otter (Algarolutra)
- Sardinian giant otter (Megalenhydris barbaricina)
- Sardinian dwarf otter (Sardolutra)
- Cretan otter (Lutrogale cretensis)
- Various Ursus spp.
- Deninger's bear (Ursus deningeri)
- Etruscan bear (Ursus etruscus)
- Gamssulzen cave bear (Ursus ingressus)[113]
- Pleistocene small cave bear (Ursus rossicus)
- Cave bear (Ursus spelaeus)
- Giant polar bear (Ursus maritimus tyrannus)
- All native Elephant (Elephantidae) spp.
- Woolly mammoth (Mammuthus primigenius)
- Dwarf mammoth
- Cretan dwarf mammoth (Mammuthus creticus)
- Dwarf Sardinian mammoth (Mammuthus lamarmorai)
- Straight-tusked elephant (Palaeoloxodon antiquus)
- Dwarf elephant
- Palaeoloxodon chaniensis
- Cyprus dwarf elephant (Palaeoloxodon cypriotes)
- Pygmy elephant (Palaeoloxodon falconeri)
- Palaeoloxodon mnaidriensis
- Balearic giant dormouse (Hypnomys) spp. e.g.
- Majorcan giant dormouse (Hypnomys morpheus)
- Leithia spp. (Maltese and Sicilian giant dormouse)[114]
- Pika (Ochotona) spp. e.g.
- Giant pika (Ochotona whartoni)
- Asian ostrich (Struthio asiaticus)
- Giant swan (Cygnus falconeri)
- Yakutian goose (Anser djuktaiensis)
- Various European crane spp. (Genus Grus)
- Grus primigenia
- Grus melitensis
- Cretan owl (Athene cretensis)
- Denisovans (Homo sp.)
- Neanderthals (Homo (sapiens) neanderthalensis; survived until about 40,000 years ago on the Iberian peninsula)
Many species extant today were present in areas either far to the south or west of their contemporary ranges- for example, all the arctic fauna on this list inhabited regions as south as the Iberian Peninsula at various stages of the Late Pleistocene. Recently extinct organisms are noted as †. Species extirpated from significant portions of or all former ranges in Europe and northern Asia during the Quaternary extinction event include-
- †European lion (Panthera leo europaea)
- Tiger (Panthera tigris, from the Ukraine Black Sea to Beringia)[115][116][117]
- Cheetah (Acinonyx jubatus)[118][119]
- Leopard (Panthera pardus)
- Snow leopard (Panthera uncia)
- Eurasian and Iberian lynx (Lynx lynx and Lynx pardinus)
- Wolverine (Gulo gulo)
- Polar bear (Ursus maritimus)
- Arctic fox (Vulpes lagopus)
- Dhole (Cuon alpinus)
- Gray wolf (†Megafaunal et Beringian wolf, and the Paleolithic dog (Canis lupus))
- †Tarpan (Equus ferus ferus)
- Fallow deer (Dama dama)
- Mouflon (Ovis gmelini)
- Chamois (Rupicapra spp.)
- West Caucasian tur (Capra caucasica)[97][98]
- Saiga antelope (Saiga tatarica)
- Reindeer (Rangifer tarandus)
- Moose (Alces alces)
- Onager (Equus hemionus)
- †Aurochs (Bos primigenius)
- European bison (Bison bonasus)
- Asian water buffalo (Bubalus arnee)[120]
- Musk ox (Ovibos moschatus)
- Asian elephant (Elephas maximus, from the Black Sea to Northern China)
- Steppe pika (Ochotona pusilla)
- Great jerboa (Allactaga major)
- Hippopotamus (Hippopotamus amphibius)
- Northern bald ibis (Geronticus eremita)
- †Great auk (Pinguinus impennis)[121]
- Snowy owl (Bubo scandiacus)
- Barbary macaque (Macaca sylvanus)
Nearctic: North America










During the last 60,000 years, including the end of the last glacial period, approximately 51 genera of large mammals have become extinct in North America. Of these, many genera extinctions can be reliably attributed to a brief interval of 11,500 to 10,000 radiocarbon years before present, shortly following the arrival of the Clovis people in North America [citation needed]. Prominent paleontological sites include Mexico[122][123][124][125] and Panama, the crossroads of the American Interchange.[126] Most other extinctions are poorly constrained in time, though some definitely occurred outside of this narrow interval.[127] In contrast, only about half a dozen small mammals disappeared during this time. Previous North American extinction pulses had occurred at the end of glaciations, but not with such an ecological imbalance between large mammals and small ones (Moreover, previous extinction pulses were not comparable to the Quaternary extinction event; they involved primarily species replacements within ecological niches, while the latter event resulted in many ecological niches being left unoccupied). Such include the last native North American terror bird (Titanis), rhinoceros (Aphelops) and hyena (Chasmaporthetes). Human habitation commenced unequivocally approximately 22,000 BCE north of the glacier,[8] and 13,500 BCE south,[128][129] however disputed evidence of southern human habitation exists from 130,000 BCE and 17,000 BCE onwards, described from sites in California and Meadowcroft in Pennsylvania.[122][130] North American extinctions (noted as herbivores (H) or carnivores (C)) included:
- Various Bovidae spp.
- Most forms of Pleistocene bison (only Bison bison in North America, and Bison bonasus in Eurasia, survived)
- Ancient bison (Bison antiquus) (H)
- Long-horned/Giant bison (Bison latifrons) (H)
- Steppe bison (Bison priscus) (H)
- Bison occidentalis (H)
- Wild yak (Bos mutus; extirpated)[131] (H)
- Several members of Caprinae (the muskox survived)
- Giant muskox (Praeovibos priscus) (H)
- Shrub-ox (Euceratherium collinum) (H)
- Harlan's muskox (Bootherium bombifrons) (H)
- Soergel's ox (Soergelia mayfieldi) (H)
- Harrington's mountain goat (Oreamnos harringtoni; smaller and more southern distribution than its surviving relative) (H)
- Saiga antelope (Saiga tatarica; extirpated) (H)
- Most forms of Pleistocene bison (only Bison bison in North America, and Bison bonasus in Eurasia, survived)
- Stag-moose (Cervalces scotti) (H)
- American mountain deer (Odocoileus lucasi) (H)
- Torontoceros hypnogeos (H)
- Various Antilocapridae genera (pronghorns survived)
- Capromeryx (H)
- Hayoceros (H)
- Stockoceros (H)
- Tetrameryx (H)
- Pacific pronghorn (Antilocapra pacifica) (H)
- Several peccary (Tayassuidae) spp.
- Flat-headed peccary (Platygonus) (H)
- Long-nosed peccary (Mylohyus) (H)
- Collared peccary (Dicotyles tajacu; extirpated, range semi-recolonised) (H) (Muknalia minimus is a junior synonym)
- Various members of Camelidae
- Western camel (Camelops hesternus) (H)
- Stilt legged llamas (Hemiauchenia ssp.) (H)
- Stout legged llamas (Palaeolama ssp.) (H)
- All native forms of Equidae
- Equus alaskae (H)
- Equus cedralensis[132] (H)
- Mexican horse (Equus conversidens) (H)
- Equus complicatus[133] (H)
- Equus fraternus (H)
- Giant horse[134] (H)
- Onager (Equus hemionus; extirpated)[135] (H)
- Kiang (Equus kiang; extirpated)[136] (H)
- Yukon horse (Equus lambei) (H)
- Equus mexicanus[137] (H)
- Niobrara horse (Equus niobrarensis) (H)
- Pacific horse (Equus pacificus)[138] (H)
- Western horse (Equus occidentalis) (H)
- Equus semiplicatus (H)
- Hagerman horse (Equus simplicidens) (H)
- Scott's horse (Equus scotti) (H)
- Stilt-legged horse (Haringtonhippus francisci / Equus francisci; may be a synonym of Mexican horse) (H)
- All members of North American tapir (Tapirus; four species)
- California tapir (Tapirus californicus) (H)
- Cope's tapir (Tapirus copei) (H)
- Merriam's tapir (Tapirus merriami) (H)
- Vero tapir (Tapirus veroensis) (H)
- Mixotoxodon[139][140] (H)
- Several Felidae spp.
- North American saber-toothed cat (Smilodon fatalis) (C)
- North American scimitar cat (Homotherium serum) (C)
- American cheetah (Miracinonyx; not true cheetah)
- Miracinonyx inexpectatus (C)
- Miracinonyx trumani (C)
- Cougar (Puma concolor; megafaunal ecomorph extirpated from North America, South American populations recolonised former range) (C)
- Jaguarundi (Herpailurus yagouaroundi; extirpated, range semi-recolonised) (C)
- Margay (Leopardus weidii; extirpated) (C)
- Ocelot (Leopardus pardalis; extirpated, range marginally recolonised) (C)
- Eurasian lynx (Lynx lynx; extirpated)[141] (C)
- Pleistocene North American jaguar (Panthera onca augusta; range semi-recolonised by other subspecies) (C)
- American lion (Panthera atrox; endemic to North America after 340,000 BP) (C)
- Eurasian cave lion (Panthera spelaea; present only as far as modern day Yukon) (C)
- Californian sea otter (Enhydra macrodonta)[142] (C)
- Steppe polecat (Mustela eversmanii; extirpated)[143] (C)
- Dire wolf (Aenocyon dirus) (C)
- Pleistocene coyote (Canis latrans orcutti) (C)
- Megafaunal wolf e.g.
- Beringian wolf (Canis lupus ssp.) (C)
- Dhole (Cuon alpinus; extirpated) (C)
- Protocyon troglodytes[144] (C)
- Short-faced skunk (Brachyprotoma obtusata)[145] (C)
- Various bear (Ursidae) spp.
- Short-faced bear (Arctodus) spp.
- Arctodus simus (C)
- Arctodus pristinus (C)
- Florida spectacled bear (Tremarctos floridanus) (C)
- South American short-faced bear (Arctotherium wingei)[122][144] (C)
- Giant polar bear (Ursus maritimus tyrannus; a possible inhabitant) (C)
- Short-faced bear (Arctodus) spp.
- Pristine mustached bat (Pteronotus (Phyllodia) pristinus) (C)
- Stock's vampire bat (Desmodus stocki) (C)
- All native spp. of Proboscidea
- American mastodon (Mammut americanum) (H)
- Pacific mastodon (Mammut pacificus) (H)
- Gomphotheriidae spp.
- Cuvieronius[146] (H)
- Stegomastodon[147] (H)
- Mammoth (Mammuthus) spp.
- Columbian mammoth (Mammuthus columbi) (H)
- Pygmy mammoth (Mammuthus exilis) (H)
- Woolly mammoth (Mammuthus primigenius) (H)
- Steller's sea cow (Hydrodamalis gigas; extirpated in North America) (H)
- Californian beaver (Castor cf. californicus)[148] (H)
- Giant beaver (Castoroides) spp.
- Neochoerus spp. e.g.
- Pinckney's capybara (Neochoerus pinckneyi) (H)
- Klein's porcupine (Erethizon kleini)[149] (H)
- All giant hutia (Heptaxodontidae) spp.
- Blunt-toothed giant hutia (Amblyrhiza inundata; could grow as large as an American black bear) (H)
- Plate-toothed giant hutia (Elasmodontomys obliquus) (H)
- Twisted-toothed mouse (Quemisia gravis) (H)
- Osborn's key mouse (Clidomys osborn's) (H)
- Xaymaca fulvopulvis (H)
- Aztlan rabbit (Aztlanolagus sp.) (H)
- Webb's marsh rabbit (Sylvilagus webbi)[150] (H)
- Giant pika (Ochotona whartoni) (H)
- All members of the Antilles monkeys (Xenotrichini)
- Jamaican monkey (Xenothrix mcgregori) (H)
- Cuban monkey (Paralouatta) (H)
- Hispaniola monkey (Antillothrix bernensis) (H)
- Insulacebus toussaintiana (H)
- Giant anteater (Myrmecophaga tridactyla; extirpated, range partially recolonised)[151][152] (C)
- All remaining ground sloth spp.
- Eremotherium (megatheriid ground sloth) (H)
- Nothrotheriops (nothrotheriid ground sloth) (H)
- Megalonychid ground sloth spp.
- Megalonyx (H)
- Nohochichak[153][154] (H)
- Xibalbaonyx[155][156] (H)
- Megalocnid Greater Antillean dwarf ground sloth spp. (some were probably at least partly arboreal)
- Acratocnus (H)
- Habanocnus (H)
- Megalocnus (H)
- Miocnus (H)
- Neocnus (H)
- Mylodontid ground sloth spp.
- Paramylodon (H)
- Glossotherium (H)
- All members of Glyptodontidae
- Glyptotherium[157] (H)
- Pachyarmatherium (H)
- Beautiful armadillo (Dasypus bellus)[158] (H)
- All Pampatheriidae spp. e.g.
- Holmesina (H)
- Bermuda flightless duck (Anas pachyscelus) (H)
- Californian flightless sea duck (Chendytes lawi) (C)
- Mexican stiff-tailed duck (Oxyura zapatima)[122] (H)
- Turkey (Meleagris) spp.
- Californian turkey (Meleagris californica) (H)
- Meleagris crassipes[122] (H)
- Various Gruiformes spp.
- All cave rail (Nesotrochis) spp. e.g.
- Antillean cave rail (Nesotrochis debooyi) (C)
- Barbados rail (Incertae sedis) (C)
- Cuban flightless crane (Antigone cubensis) (H)
- La Brea crane (Grus pagei) (H)
- All cave rail (Nesotrochis) spp. e.g.
- Various flamingo (Phoenicopteridae) spp.
- Minute flamingo (Phoenicopterus minutus)[159] (C)
- Cope's flamingo (Phoenicopterus copei)[160] (C)
- Dow's puffin (Fratercula dowi) (C)
- Pleistocene Mexican diver spp.
- La Brea/Asphalt stork (Ciconia maltha)[122] (C)
- Wetmore's stork (Mycteria wetmorei)[122] (C)
- Pleistocene Mexican cormorants spp. (genus Phalacrocorax)[122]
- Phalacrocorax goletensis (C)
- Phalacrocorax chapalensis (C)
- Jamaican ibis (Xenicibis xympithecus) (C)
- All remaining teratorn (Teratornithidae) spp.
- Several New World vultures (Cathartidae) spp.
- Pleistocene black vulture (Coragyps occidentalis ssp.) (C)
- Megafaunal Californian condor (Gymnogyps amplus) (C)
- Clark's condor (Breagyps clarki) (C)
- Cuban condor (Gymnogyps varonai) (C)
- Several Accipitridae spp.
- American neophrone vulture (Neophrontops americanus)[122][161] (C)
- Woodward's eagle (Amplibuteo woodwardi) (C)
- Cuban great hawk (Buteogallus borrasi) (C)
- Daggett's eagle (Buteogallus daggetti) (C)
- Fragile eagle (Buteogallus fragilis) (C)
- Cuban giant hawk (Gigantohierax suarezi)[162][163] (C)
- Errant eagle (Neogyps errans) (C)
- Grinnell's crested eagle (Spizaetus grinnelli)[122] (C)
- Willett's hawk-eagle (Spizaetus willetti)[122] (C)
- Caribbean titan hawk (Titanohierax) (C)
- Several owl (Strigiformes) spp.
- Brea miniature owl (Asphaltoglaux) (C)
- Kurochkin's pygmy owl (Glaucidium kurochkini) (C)
- Brea owl (Oraristix brea) (C)
- Cuban giant owl (Ornimegalonyx) (C)
- Bermuda flicker (Colaptes oceanicus) (C)
- Several caracara (Caracarinae) spp.
- Bahaman terrestrial caracara (Caracara sp.) (C)
- Puerto Rican terrestrial caracara (Caracara sp.) (C)
- Jamaican caracara (Carcara tellustris) (C)
- Cuban caracara (Milvago sp.) (C)
- Hispaniolan caracara (Milvago sp.) (C)
- Saint Croix macaw (Ara autocthones) (H)
- Mexican thick-billed parrot (Rhynchopsitta phillipsi)[122] (H)
- Puerto Rican crow (Corvus pumilis) (C)
- Several giant tortoise spp.
- Hesperotestudo (H)
- Gopherus spp.
- Gopherus donlaloi (H)
- Chelonoidis spp.
- Chelonoidis marcanoi (H)
- Chelonoidis alburyorum (H)
The survivors are in some ways as significant as the losses: bison (H), grey wolf (C), lynx (C), grizzly bear (C), American black bear (C), deer (e.g. caribou, moose, wapiti (elk), Odocoileus spp.) (H), pronghorn (H), white-lipped peccary (H), muskox (H), bighorn sheep (H), and mountain goat (H); the list of survivors also include species which were extirpated during the Quaternary extinction event, but recolonised at least part of their ranges during the mid-holocene from South American relict populations, such as the cougar (C), jaguar (C), giant anteater (C), collared peccary (H), ocelot (C) and jaguarundi (C). All save the pronghorns and giant anteaters were descended from Asian ancestors that had evolved with human predators.[164] Pronghorns are the second-fastest land mammal (after the cheetah), which may have helped them elude hunters. More difficult to explain in the context of overkill is the survival of bison, since these animals first appeared in North America less than 240,000 years ago and so were geographically removed from human predators for a sizeable period of time.[165][166][167] Because ancient bison evolved into living bison,[168][169] there was no continent-wide extinction of bison at the end of the Pleistocene (although the genus was regionally extirpated in many areas). The survival of bison into the Holocene and recent times is therefore inconsistent with the overkill scenario. By the end of the Pleistocene, when humans first entered North America, these large animals had been geographically separated from intensive human hunting for more than 200,000 years. Given this enormous span of geologic time, bison would almost certainly have been very nearly as naive as native North American large mammals.
The culture that has been connected with the wave of extinctions in North America is the paleo-American culture associated with the Clovis people (q.v.), who were thought to use spear throwers to kill large animals. The chief criticism of the "prehistoric overkill hypothesis" has been that the human population at the time was too small and/or not sufficiently widespread geographically to have been capable of such ecologically significant impacts. This criticism does not mean that climate change scenarios explaining the extinction are automatically to be preferred by default, however, any more than weaknesses in climate change arguments can be taken as supporting overkill. Some form of a combination of both factors could be plausible, and overkill would be a lot easier to achieve large-scale extinction with an already stressed population due to climate change.
Neotropic: South America



The Neotropical realm was affected by the fact that South America had been isolated as an island continent for many millions of years, and had a wide range of fauna found nowhere else, although many of them became extinct during the Great American Interchange about 3 million years ago, such as the Sparassodonta family. Those that survived the interchange included the ground sloths, glyptodonts, litopterns, pampatheres, phorusrhacids (terror birds) and notoungulates; all managed to extend their range to North America.[170][171] [172] In the Pleistocene, South America remained largely unglaciated except for increased mountain glaciation in the Andes, which had a two-fold effect- there was a faunal divide between the Andes,[173][174] and the colder, arid interior resulted in the advance of temperate lowland woodland, tropical savanna and desert at the expense of rainforest.[175][176][177][178][179] Within these open environments, megafauna diversity was extremely dense, with over 40 genera recorded from the Guerrero member of Luján Formation alone.[180][181][182][183] Ultimately, by the mid-Holocene, all the preeminent genera of megafauna became extinct- the last specimens of Doedicurus and Toxodon have been dated to 4,555 BCE and 3,000 BCE respectively.[184][185][186][175] Their smaller relatives remain, including anteaters, tree sloths, armadillos; New World marsupials: opossums, shrew opossums, and the monito del monte (actually more related to Australian marsupials).[187] Intense human habitation was established circa 11,000 BCE, however partly disputed evidence of pre-clovis habitation occurs since 46,000 BCE and 20,000 BCE, such as at the Serra da Capivara National Park (Brazil) and Monte Verde (Chile) sites.[122][129][188] Today the largest land mammals remaining in South America are the wild camels of the Lamini group, such as the guanacos and vicuñas, and the genus Tapirus, of which Baird's tapir can reach up to 400 kg. Other notable surviving large fauna are peccaries, marsh deer (Capreolinae), giant anteaters, spectacled bears, maned wolves, pumas, ocelots, jaguars, rheas, emerald tree boas, boa constrictors, anacondas, American crocodiles, caimans, and giant rodents such as capybaras.
- Several Cervidae spp.
- Morenelaphus
- Antifer
- Agalmaceros blicki[189][190]
- Odocoileus salinae[191][184]
- Various Camelidae spp.
- Eulamaops
- Stilt legged llama Hemiauchenia
- Stout legged llama Palaeolama
- All Pleistocene wild horse genera (Equidae)
- All remaining Meridiungulata genera
- Litopterna spp.
- Macrauchenia
- Macraucheniopsis[194][195]
- Proterotheriidae spp. e.g.
- Xenorhinotherium
- Notoungulata spp.
- Hegetotheriidae spp.
- Mesotheriidae spp.
- Mixotoxodon
- Toxodon
- Litopterna spp.
- Several Felidae spp.
- Saber-toothed cat (Smilodon) spp.[173]
- North American saber-toothed cat (Smilodon fatalis)
- South American saber-toothed cat (Smilodon populator)
- Pleistocene South American jaguar (Panthera onca mesembrina)
- Saber-toothed cat (Smilodon) spp.[173]
- Dire wolf (Aenocyon dirus)
- Nehring's wolf (Canis nehringi)
- Protocyon spp.[197]
- Protocyon trogolodytes
- Protocyon tarijense
- Dusicyon avus
- Pleistocene bush dog (Speothos pacivorus)
- South American short-faced bear (Arctotherium spp.)
- Arctotherium bonairense
- Arctotherium tarijense
- Arctotherium wingei
- Giant vampire bat (Desmodus draculae)
- All remaining Gomphotheridae spp.
- Neochoerus
- All remaining ground sloth genera
- Megatheriidae spp.
- Nothrotheriidae spp.
- Megalonychidae spp.
- Mylodontidae spp.
- All remaining Glyptodontinae spp.
- Several Dasypodidae spp.
- Beautiful armadillo (Dasypus bellus)
- Eutatus
- Pachyarmatherium
- Propaopus[105][184]
- All Pampatheriidae spp.
- Holmesina (et 'Chlamytherium occidentale')[206][207]
- Pampatherium[208]
- Tonnicinctus[208]
- Psilopterus (small terror bird remains dated to the Late Pleistocene,[209][210] but these are disputed)[211]
- Various Caracarinae spp.
- Various Cathartidae spp.
- Pampagyps imperator
- Geronogyps reliquus
- Wingegyps cartellei
- Pleistovultur nevesi
- Caiman venezuelensis
- Chelonoidis lutzae (Argentina)
The Pacific (Australasia and Oceania)
- One thing that makes the extinction of the Australian megafauna particularly special is that prior to and into the Quaternary period the vast oceans prevented homo sapiens and other animals from reaching the outer world: islands like Australia and Madagascar. As a result, organisms in these places evolved in isolation for millions of years taking on quite different structures from their Afro-Asian relatives. One key characteristic of the Australian animals is that they were marsupials. Marsupials are animals that give birth to tiny, helpless offspring and then nurture them with breast milk in their abdominal pouches. While many of the animals of Australia were marsupial these types of animals were almost unknown in Africa and Asia. Secondly many of the animals in Australia were megafauna, megafauna are animals that weight 100 pounds or more. During the quaternary period homo sapiens acquired the technology to sail the oceans allowing them to settle the outer world. Evidence shows that homo sapiens first arrived in Australia about 70,000 year ago and colonizing the landscape in about 30,000 years. Within this 30,000-year period 90% of megafauna species inhabiting Australia went extinct. Scientists hold three main theories behind the extinction of the Australian megafauna. One is that it was caused by overkill after the widespread appearance of homo sapiens, another is that they went extinct due to natural climate change, and the final theory is that extinction was caused by a combination of overkill by humans and natural climate change.[215]
Beginning with the idea of a human caused extinction event from the book Sapiens by Yuval Noah Harari, there exists three pieces of evidence that support this hypothesis. First, despite Australia’s climate changing at the time it is hard to say natural climate change alone could have caused such a massive extinction event. For example, the giant diprotodon inhabited Australia for more than 1.5 million years before humans showed up and the species is known to have survived 10 previous ice ages. So, if the giant diproton had survived 10 previous ice ages why did it along with 90% of the other megafauna die out around 45,000 years ago around when homo sapiens colonized Australia. Secondly, typically when climate change is the cause of mass extinctions, sea organisms usually experience greater consequences than organisms living on land. Yet there is no evidence of any significant extinction of oceanic fauna 45,000 years ago in Australia. This points to homo sapiens being the cause of the extinction of the Australian megafauna because at the time homo sapiens still overwhelmingly lived off the land as opposed to the sea. Thirdly the extinction of megafauna following the arrival of humans has been seen in multiple outer world locations over the last millennia. Examples include New Zealand only 800 years ago and Wrangel, an artic island, 4000 years ago. Therefore, it makes sense that this pattern would also hold true in the case of Australian megafauna.[215]
Given that 90% of Australian megafauna went extinct within 30,000 years of the arrival of human beings one must ask themselves why these animals were not fit to survive against humans. One of these reasons is that megafauna are exceptionally large animals. As a result, these animals breed slowly, have long pregnancies periods, and produce few offspring per pregnancy. In addition, there is usually a long break in between pregnancies. Secondly many of these animals were marsupials so there existed a long development period from birth to maturity and offspring were dependent on their parents for breastmilk for quite some time. These two characteristics combined contributed to the extinction of Australian megafauna because hunting is more detrimental to these types of populations than others. For example, if human beings killed one diprotodon every few months it would be enough for yearly diprotodon deaths to outnumber diprotodon births causing population decline, and within a thousand years this could lead to extinction. Another aspect making Australian megafauna less fit to survive against humans is that homo sapiens evolved separately from the Australian megafauna, so the megafauna had no predeveloped fear towards humans making hunting them easier.[215]
The second hypothesis held by scientists is that the Australian megafauna went extinct due to natural climate change. The main reason this theory exists is that there is evidence of megafauna surviving up until 40,000 years ago, a full 30,000 years after homo sapiens first landed in Australia. Implying that there was a significant period of homo sapiens and megafauna coexistence. Evidence of these animals existing at this time come from fossils records and ocean sediment. To begin with, sediment core drilled in the Indian Ocean off the coast of the southwest Australia indicate the existence of a fungus called Sporormiella which survived off the dung of plant eating mammals. The abundance of these spores in the sediment prior to 45,000 years ago indicates a lot of large mammals existed on the southwest Australian landscape up until that point. The sediment data also indicated that the megafauna population collapsed within a few thousand years around the 45,000 years ago suggesting a rapid extinction event[216]. In addition, fossils found at South Walker Creek, which is the youngest megafauna site in northern Australia, indicate that at least 16 species of megafauna survived there up until 40,000 years ago. Furthermore, there is no firm evidence of homo sapiens beings at South Walker Creek 40,000 years ago, therefore no human cause can be attributed to the extinction of these megafauna. However, there is evidence of major environmental deterioration of South Water Creek 40,000 years ago which the extinction can be attributed to. These changes include increased fire, reduction in grasslands, and the loss of freshwater[217]. The same environmental deterioration is seen across Australia at the time further strengthening the climate change argument. Australia’s climate at the time could best be described as an overall drying of the landscape due to less mean annual precipitation causing less freshwater availability and more drought conditions across the landscape. Overall, this led to changes in vegetation, increased fires, overall reduction in grasslands, and a greater competition for already scarce amount of freshwater[218]. In turn all these environmental changes proved to me too much for the Australian megafauna to cope with causing 90% of megafauna species to go extinct.
The third hypothesis shared by some scientists is that human impacts and natural climate changes led to the extinction of Australian megafauna. To begin with it is important to note that approximately 75% of Australia is semi-arid or arid landscape, therefore it makes sense that megafauna species utilized the same freshwater resources as humans. As a result, this could have increased the amount of megafauna hunted due to the competition for freshwater as the drought conditions persisted[219]. On top of the already dry conditions and diminishing grasslands, homo sapiens used fire agriculture to burn impassable land. This further diminished the already disappearing grassland which contained plants that were key dietary component of herbivorous megafauna. While there is no scientific consensus on the true cause of the extinction of Australian megafauna it is plausible that homo sapiens and natural climate change both had an impact because they were both in Australia at the time. Overall, there is an immense amount of evidence pointing to humans being the culprit but by ruling out climate change completely as a cause of the Australian megafauna extinction we are not getting the whole picture. The climate change that occurred in Australia 45,000 years ago destabilized the ecosystem making it particularly vulnerable to hunting and fire agriculture by humans; this is probably what led to the extinction of the Australian megafauna.


In Sahul (a former continent composed of Australia and New Guinea), the sudden and extensive spate of extinctions occurred earlier than in the rest of the world.[220][221][222][223][224] Most evidence points to a 20,000 year period after human arrival circa 63,000 BCE,[7] but scientific argument continues as to the exact date range.[225] In the rest of the Pacific (other Australasian islands such as New Caledonia, and Oceania) although in some respects far later, endemic fauna also usually perished quickly upon the arrival of humans in the late Pleistocene and early Holocene. This section does only include extinctions that took place prior to European discovery of the respective islands.
The extinctions in the Pacific included:
- Various members of Diprotodontidae
- Diprotodon
- Euowenia
- Euryzygoma dunense
- Hulitherium tomasetti
- Maokopia ronaldi
- Nototherium
- Zygomaturus
- Palorchestes ("marsupial tapir")
- Various members of Vombatidae
- Phascolarctos stirtoni (giant koala)
- Marsupial lion (Thylacoleo carnifex)
- Various members of Macropodidae
- Procoptodon (short-faced kangaroos) e.g.
- Procoptodon goliah
- Sthenurus (giant kangaroo)
- Simosthenurus (giant kangaroo)
- Various Macropus (giant kangaroo) spp. e.g.
- Protemnodon (giant wallaby)
- Troposodon (wallaby)[221][222][226][227][228][229]
- Bohra (giant tree kangaroo)
- Propleopus oscillans (omnivorous, giant musky rat-kangaroo)
- Procoptodon (short-faced kangaroos) e.g.
- Thylacine (Thylacinus cynocephalus; extirpated on mainland Australia and New Guinea)
- Various forms of Sarcophilus (Tasmanian devil)
- Sarcophilus laniarius (25 % larger than modern species)
- Sarcophilus moornaensis
- Sarcophilus harrisii (extirpated on mainland Australia)
- Zaglossus hacketti (giant echidna)
- Megalibgwilia (oldest known echidna, same extinction period)
- Pygmy Cassowary (Casuarius lydekkeri)
- Mihirung (a three-meter-tall (9.8 ft) dromornithid
- Tasmanian nativehen (Tribonyx mortierii; extirpated on mainland Australia)
- Giant malleefowl (Leipoa gallinacea)
- Several Phoenicopteridae spp.
- American flamingo (Phoenicopterus ruber; extirpated in Australia)[231]
- Xenorhynchopsis spp. (Australian flamingo)[231]
- Xenorhynchopsis minor
- Xenorhynchopsis tibialis
- Ocyplanus proeses (Australian flamingo)[231]
- Ikanogavialis (the last fully marine crocodilian)
- Pallimnarchus (Australian freshwater crocodile)
- Quinkana (Australian terrestrial crocodile, apex predator)
- Wonambi (a five-to-six-metre-long Australian constrictor snake)
- Megalania (Varanus pricus) (a giant predatory monitor lizard)
- Several spp. of Meiolaniidae (giant armoured tortoises)
- Sylviornis (giant, flightless New Caledonian galliform; largest in existence)
- Noble megapode (Megavitornis altirostris)
- Giant Megapodius spp.
- Pile-builder megapode (Megapodius molistructor)
- Consumed scrubfowl (Megapodius alimentum)
- Viti Levu scrubfowl (Megapodius amissus)
- New Caledonian ground dove (Gallicolumba longitarsus)
- Viti Levu giant pigeon (Natunaornis gigoura)
- Marquesas cuckoo-dove (Macropygia heana)
- New Caledonian gallinule (Porphyrio kukwiedei)
- Various Gallirallus spp.
- Various Coenocorypha spp.
- New Caledonian snipe (Coenocorypha miratropica)
- Viti Levu snipe (Coenocorypha neocaledonica)
- Lowland kagu (Rhynochetos orarius)
- Niue night heron (Nycticorax kalavikai)
- Several Accipiter spp.[3]
- Powerful goshawk (Accipiter efficax)
- Gracile goshawk (Accipiter quartus)
- New Caledonian barn owl (Tyto letocarti)
- Mekosuchus (two meters long, last fully terrestrial crocodile, South Pacific Islands)
- Volia (a two-to-three meter long mekosuchine crocodylian, apex predator of Pleistocene Fiji)
- Varanus sp. (Pleistocene and Holocene New Caledonia)
- Several giant Iguanidae spp.
- All Dinornithiformes spp.
- Giant moa (Dinornis)
- Upland moa (Megalapteryx didinus)
- Bush moa (Anomalopteryx didiformis)
- Eastern moa (Emeus crassus)
- Coastal moa (Euryapteryx curtus)
- Pachyornis
- Scarlett's duck (Malacorhynchus scarletti)
- New Zealand musk duck (Biziura delautouri)
- Chatham Islands duck (Pachyanas chathamica)
- New Zealand goose (Cnemiornis)
- New Zealand swan (Cygnus sumnerensis)
- New Zealand owlet-nightjar (Aegotheles novazelandiae)
- Adzebill (Aptornis)
- Snipe-rail (Capellirallus karamu)
- Hodgen's waterhen (Gallinula hodgenorum)
- Waitaha penguin (Megadyptes waitaha)
- Scarlett's shearwater (Puffinus spelaeus)
- Several harriers (Circus)
- Eyles's harrier (Circus eylesi)
- Wood harrier (Circus dossenus; endemic to Hawaii)
- Haast's eagle (Hieraaetus moorei; largest eagle known to have existed)
- Various Corvus spp.
- New Zealand raven (Corvus antipodum)
- Chatham raven (Corvus moriorum)
- High-billed crow (Corvus impluviatus; large crow endemic to Maui)
- Long-billed wren (Dendroscansor decurvirostris)
- Stout-legged wren (Pachyplichas yaldwyni)
- Kawekaweau (Hoplodactylus delcourti)
- Northland skink (Oligosoma northlandi)
- Several frogs of the genus Leiopelma
- Aurora frog (Leiopelma auroraensis)
- Markham's frog (Leiopelma markhami)
- Waitomo frog (Leiopelma waitomoensis)
- Synemporion keana (Bat endemic to Hawaii)
- Kaua'i mole duck (Talpanas lippa; a blind, flightless, terrestrial Hawaiian duck)
- All members of Thambetochenini
- Turtle-jawed moa-nalo (Chelychelynechen quassus; from Kaua'i)
- Small-billed moa-nalo (Ptaiochen pau; from Maui)
- O'ahu moa-nalo (Thambetochen xanion)
- Maui Nui large-billed moa-nalo (Thambetochen chauliodous)
- Giant Hawaii goose (Branta rhuax)
- Nēnē-nui (Branta hylobadistes)
- Great Maui crake (Porzana severnsi)
- O'ahu petrel (Pterodroma jugabilis)
- Apteribis (a giant, flightless ibis)
- Stilt-owl (Grallistrix)
- Giant nukupu'u (Hemignathus vorpalis)
- Stout-legged finch (Ciridops tenax)
- Several finches of the genus Telespiza
- Kaua'i finch (Telespiza persecutrix)
- Maui Nui finch (Telespiza ypsilon)
- Kaua'i palila (Loxioides kikuchi)
- Several Rhodacanthis spp.
- Primitive koa finch (Rhodacanthis litotes)
- Scissor-billed koa finch (Rhodacanthis forfex)
- O'ahu grosbeak (Chloridops wahi)
- Easter Island crake (Porzana sp.)
- Easter Island rail (undescribed)
- Undescribed Easter Island heron
- Barn owl (Tyto alba; extirpated on Easter Island)
- Two species of undescribed Easter Island parrots
Some extinct megafauna, such as the bunyip-like Diprotodon, may remain in folk memory or be the sources of cryptozoological legends.
Relationship to later extinctions
There is no general agreement on where the Holocene, or anthropogenic, extinction begins, and the Quaternary extinction event ends, or if they should be considered separate events at all.[232][233] Some have suggested that anthropogenic extinctions may have begun as early as when the first modern humans spread out of Africa between 100,000 and 200,000 years ago, which is supported by rapid megafaunal extinction following recent human colonisation in Australia, New Zealand and Madagascar,[234] in a similar way that any large, adaptable predator moving into a new ecosystem would. In many cases, it is suggested even minimal hunting pressure was enough to wipe out large fauna, particularly on geographically isolated islands.[235][236] Only during the most recent parts of the extinction have plants also suffered large losses.[237]
Overall, the Holocene extinction can be characterised by the human impact on the environment. The Holocene extinction continues into the 21st century, with overfishing, ocean acidification and the amphibian crisis being a few broader examples of an almost universal, cosmopolitan decline of biodiversity.
Hunting hypothesis
The hunting hypothesis suggests that humans hunted megaherbivores to extinction, which in turn caused the extinction of carnivores and scavengers which had preyed upon those animals.[238][239][240] Therefore, this hypothesis holds Pleistocene humans responsible for the megafaunal extinction. One variant, known as blitzkrieg, portrays this process as relatively quick. Some of the direct evidence for this includes: fossils of some megafauna found in conjunction with human remains, embedded arrows and tool cut marks found in megafaunal bones, and European cave paintings that depict such hunting. Biogeographical evidence is also suggestive: the areas of the world where humans evolved currently have more of their Pleistocene megafaunal diversity (the elephants and rhinos of Asia and Africa) compared to other areas such as Australia , the Americas, Madagascar and New Zealand without the earliest humans. A picture arises of the megafauna of Asia and Africa evolving alongside humans, learning to be wary of them, and in other parts of the world the wildlife appearing ecologically naive and easier to hunt.[citation needed] This is particularly true of island fauna, which display a disastrous lack of fear of humans.[citation needed] Of course, it is impossible to demonstrate this naïveté directly in ancient fauna. It is highly assumed that despite many large animals easily viewing humans as a threat, humans' endurance, by literally endlessly chasing said animals over long distances, something most animals, even fast predators, are incapable of doing so, combined with the domestication of dogs, as wolves are also known to have endurance, ultimately made these animals more vulnerable to extinction, as such ended up overwhelming them, and therefore making them too weak to even defend themselves. However, even after the development of weapons used to kill prey much faster as well as domesticating dogs ultimately the defeated the purpose of early humans simply using endurance to catch prey, this only ended up making the resulting extinctions even more severe.
Circumstantially, the close correlation in time between the appearance of humans in an area and extinction there provides weight for this scenario. The megafaunal extinctions covered a vast period of time and highly variable climatic situations. The earliest extinctions in Australia were complete approximately 50,000 BP, well before the last glacial maximum and before rises in temperature. The most recent extinction in New Zealand was complete no earlier than 500 BP and during a period of cooling. In between these extremes megafaunal extinctions have occurred progressively in such places as North America, South America and Madagascar with no climatic commonality. The only common factor that can be ascertained is the arrival of humans.[241][242] This phenomenon appears even within regions. The mammal extinction wave in Australia about 50,000 years ago coincides not with known climatic changes, but with the arrival of humans. In addition, large mammal species like the giant kangaroo Protemnodon appear to have succumbed sooner on the Australian mainland than on Tasmania, which was colonised by humans a few thousand years later.[243][244]
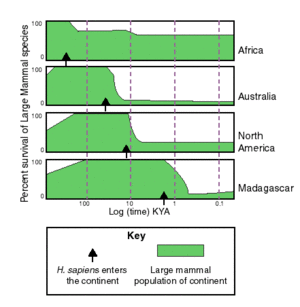
Worldwide, extinctions seem to follow the migration of humans and to be most severe where humans arrived most recently and least severe where humans originated — in Africa (see figure "March of Man" below). This suggests that prey animals and human hunting ability evolved together, so the animals evolved avoidance techniques. As humans migrated throughout the world and became more and more proficient at hunting, they encountered animals that had evolved without the presence of humans. Lacking the fear of humans that African animals had developed, animals outside of Africa were easy prey for human hunting techniques. It also suggests that this is independent of climate change.Template:Citation requested
Extinction through human hunting has been supported by archaeological finds of mammoths with projectile points embedded in their skeletons, by observations of modern naïve animals allowing hunters to approach easily[245][246][247] and by computer models by Mosimann and Martin,[248] and Whittington and Dyke,[249] and most recently by Alroy.[250]
A study published in 2015 supported the hypothesis further by running several thousand scenarios that correlated the time windows in which each species is known to have become extinct with the arrival of humans on different continents or islands.[251] This was compared against climate reconstructions for the last 90,000 years.[251] The researchers found correlations of human spread and species extinction indicating that the human impact was the main cause of the extinction, while climate change exacerbated the frequency of extinctions.[251][252] The study, however, found an apparently low extinction rate in the fossil record of mainland Asia.[252]
Overkill hypothesis
The overkill hypothesis, a variant of the hunting hypothesis, was proposed in 1966 by Paul S. Martin, Professor of Geosciences Emeritus at the Desert Laboratory of the University of Arizona.[253]
Objections to the hunting hypothesis
The major objections to the theory are as follows:
- In predator-prey models it is unlikely that predators could over-hunt their prey, since predators need their prey as food to sustain life and to reproduce.[254] This assumes that all food sources die out simultaneously, but humans could have made the mammoth extinct while subsisting on elk, for example. Human hunting is known to have exterminated megafauna on several islands, switching to other food sources with time or dying out themselves. Additionally it is common knowledge among ornithologists that introduced predators have easily made several species extinct on islands, and this is a foremost cause of island extinctions today.
- There is no archeological evidence that in North America megafauna other than mammoths, mastodons, gomphotheres and bison were hunted, despite the fact that, for example, camels and horses are very frequently reported in fossil history.[255] Overkill proponents, however, say this is due to the fast extinction process in North America and the low probability of animals with signs of butchery to be preserved.[256] Additionally, biochemical analyses have shown that Clovis tools were used in butchering horses and camels.[257] A study by Surovell and Grund[258] concluded "archaeological sites dating to the time of the coexistence of humans and extinct fauna are rare. Those that preserve bone are considerably more rare, and of those, only a very few show unambiguous evidence of human hunting of any type of prey whatsoever."
- A small number of animals that were hunted, such as a single species of bison, did not go extinct. This cannot be explained by proposing that surviving bison in North America were recent Eurasian immigrants that were familiar with human hunting practices, since Bison first appeared in North America approximately 240,000 years ago[165][166][167] and then evolved into living bison.[168][259] Bison at the end of the Pleistocene were thus likely to have been almost as naive as their native North American megafaunal companions.
- The dwarfing of animals is not explained by overkill. Numerous authors[who?], however, have pointed out that dwarfing of animals is perfectly well explained by humans selectively harvesting the largest animals, and have provided proof that even within the 20th century numerous animal populations have reduced in average size due to human hunting.
- Eurasian Pleistocene megafauna became extinct in roughly same time period despite having a much longer time to adapt to hunting pressure by humans. However, the extinction of the Eurasian megafauna can be viewed as a result of a different process than that of the American megafauna. This makes the theory less parsimonious since another mechanism is required. The latter case occurred after the sudden appearance of modern human hunters on a land mass they had never previously inhabited, while the former case was the culmination of the gradual northward movement of human hunters over thousands of years as their technology for enduring extreme cold and bringing down big game improved. Thus, while the hunting hypothesis does not necessarily predict the rough simultaneity of the north Eurasian and American megafaunal extinctions, this simultaneity cannot be regarded as evidence against it.
- Eugene S. Hunn points out that the birthrate in hunter-gatherer societies is generally too low, that too much effort is involved in the bringing down of a large animal by a hunting party, and that in order for hunter-gatherers to have brought about the extinction of megafauna simply by hunting them to death, an extraordinary amount of meat would have had to have been wasted.[260] It is possible that those who advocate the overkill hypothesis simply have not considered the differences in outlook between typical forager (hunter-gatherer) cultures and the present-day industrial cultures which exist in modernized human societies; waste may be tolerated in the latter, but is not so much in the former. Conversely, "buffalo jumps"[261] featured indiscriminate killing of a herd. However, Hunn's comments are in reference to the now largely discredited theory of hunter-prey equilibrium reached after thousands of years of coexistence. It is not relevant to hunters newly arrived on a virgin land mass full of easily taken big game. The well-established practice of industrial-scale moa butchering by the early Maori, involving enormous wastage of less choice portions of the meat, indicates that these arguments are incorrect.[245]
- The hypothesis that the Clovis culture represented the first humans to arrive in the New World has been disputed recently. (See Settlement of the Americas.) However, Clovis artifacts are currently the earliest-known evidence of widespread settlement in the Americas.
Climate change hypothesis
At the end of the 19th and beginning of the 20th centuries, when scientists first realized that there had been glacial and interglacial ages, and that they were somehow associated with the prevalence or disappearance of certain animals, they surmised that the termination of the Pleistocene ice age might be an explanation for the extinctions.
Critics object that since there were multiple glacial advances and withdrawals in the evolutionary history of many of the megafauna, it is rather implausible that only after the last glacial maximum would there be such extinctions. However, this criticism is rejected by a recent study indicating that terminal Pleistocene megafaunal community composition may have differed markedly from faunas present during earlier interglacials, particularly with respect to the great abundance and geographic extent of Pleistocene Bison at the end of the epoch.[262] This suggests that the survival of megafaunal populations during earlier interglacials is essentially irrelevant to the terminal Pleistocene extinction event, because bison were not present in similar abundance during any of the earlier interglacials.
Some evidence weighs against climate change as a valid hypothesis as applied to Australia. It has been shown that the prevailing climate at the time of extinction (40,000–50,000 BP) was similar to that of today, and that the extinct animals were strongly adapted to an arid climate. The evidence indicates that all of the extinctions took place in the same short time period, which was the time when humans entered the landscape. The main mechanism for extinction was probably fire (started by humans) in a then much less fire-adapted landscape. Isotopic evidence shows sudden changes in the diet of surviving species, which could correspond to the stress they experienced before extinction.[263][264][265]
Evidence in Southeast Asia, in contrast to Europe, Australia, and the Americas, suggests that climate change and an increasing sea level were significant factors in the extinction of several herbivorous species. Alterations in vegetation growth and new access routes for early humans and mammals to previously isolated, localized ecosystems were detrimental to select groups of fauna.[266]
Some evidence obtained from analysis of the tusks of mastodons from the American Great Lakes region appears inconsistent with the climate change hypothesis. Over a span of several thousand years prior to their extinction in the area, the mastodons show a trend of declining age at maturation. This is the opposite of what one would expect if they were experiencing stresses from deteriorating environmental conditions, but is consistent with a reduction in intraspecific competition that would result from a population being reduced by human hunting.[267]
Increased temperature
The most obvious change associated with the termination of an ice age is the increase in temperature. Between 15,000 BP and 10,000 BP, a 6 °C increase in global mean annual temperatures occurred. This was generally thought to be the cause of the extinctions.
According to this hypothesis, a temperature increase sufficient to melt the Wisconsin ice sheet could have placed enough thermal stress on cold-adapted mammals to cause them to die. Their heavy fur, which helps conserve body heat in the glacial cold, might have prevented the dumping of excess heat, causing the mammals to die of heat exhaustion. Large mammals, with their reduced surface area-to-volume ratio, would have fared worse than small mammals.
A study covering the past 56,000 years indicates that rapid warming events with temperature changes of up to 16 °C (29 °F) had an important impact on the extinction of megafauna. Ancient DNA and radiocarbon data indicates that local genetic populations were replaced by others within the same species or by others within the same genus. Survival of populations was dependent on the existence of refugia and long distance dispersals, which may have been disrupted by human hunters.[268]
Arguments against the temperature hypothesis
Studies propose that the annual mean temperature of the current interglacial that we have seen for the last 10,000 years is no higher than that of previous interglacials, yet some of the same large mammals survived similar temperature increases. Therefore, warmer temperatures alone may not be a sufficient explanation.[269][270][271][272][273][274]
In addition, numerous species such as mammoths on Wrangel Island[275] and St. Paul Island survived in human-free refugia despite changes in climate. This would not be expected if climate change were responsible (unless their maritime climates offered some protection against climate change not afforded to coastal populations on the mainland). Under normal ecological assumptions island populations should be more vulnerable to extinction due to climate change because of small populations and an inability to migrate to more favorable climes.Template:Citation requested
Increased continentality affects vegetation in time or space
Other scientists have proposed that increasingly extreme weather—hotter summers and colder winters—referred to as "continentality", or related changes in rainfall caused the extinctions. The various hypotheses are outlined below.
Vegetation changes: geographic
It has been shown that vegetation changed from mixed woodland-parkland to separate prairie and woodland.[271][272][274] This may have affected the kinds of food available. Shorter growing seasons may have caused the extinction of large herbivores and the dwarfing of many others. In this case, as observed, bison and other large ruminants would have fared better than horses, elephants and other monogastrics, because ruminants are able to extract more nutrition from limited quantities of high-fiber food and better able to deal with anti-herbivory toxins.[276][277][278] So, in general, when vegetation becomes more specialized, herbivores with less diet flexibility may be less able to find the mix of vegetation they need to sustain life and reproduce, within a given area.
Rainfall changes: time
Increased continentality resulted in reduced and less predictable rainfall limiting the availability of plants necessary for energy and nutrition.[279][280][281] Axelrod[282] and Slaughter[283] have suggested that this change in rainfall restricted the amount of time favorable for reproduction. This could disproportionately harm large animals, since they have longer, more inflexible mating periods, and so may have produced young at unfavorable seasons (i.e., when sufficient food, water, or shelter was unavailable because of shifts in the growing season). In contrast, small mammals, with their shorter life cycles, shorter reproductive cycles, and shorter gestation periods, could have adjusted to the increased unpredictability of the climate, both as individuals and as species which allowed them to synchronize their reproductive efforts with conditions favorable for offspring survival. If so, smaller mammals would have lost fewer offspring and would have been better able to repeat the reproductive effort when circumstances once more favored offspring survival.[284]
In 2017 a study looked at the environmental conditions across Europe, Siberia and the Americas from 25,000–10,000 YBP. The study found that prolonged warming events leading to deglaciation and maximum rainfall occurred just prior to the transformation of the rangelands that supported megaherbivores into widespread wetlands that supported herbivore-resistant plants. The study proposes that moisture-driven environmental change led to the megafaunal extinctions and that Africa's trans-equatorial position allowed rangeland to continue to exist between the deserts and the central forests, therefore fewer megafauna species became extinct there.[268]
Arguments against the continentality hypotheses
Critics have identified a number of problems with the continentality hypotheses.
- Megaherbivores have prospered at other times of continental climate. For example, megaherbivores thrived in Pleistocene Siberia, which had and has a more continental climate than Pleistocene or modern (post-Pleistocene, interglacial) North America.[285][286][287]
- The animals that became extinct actually should have prospered during the shift from mixed woodland-parkland to prairie, because their primary food source, grass, was increasing rather than decreasing.[288][287][289] Although the vegetation did become more spatially specialized, the amount of prairie and grass available increased, which would have been good for horses and for mammoths, and yet they became extinct. This criticism ignores the increased abundance and broad geographic extent of Pleistocene Bison at the end of the Pleistocene, which would have increased competition for these resources in a manner not seen in any earlier interglacials.[262]
- Although horses became extinct in the New World, they were successfully reintroduced by the Spanish in the 16th century—into a modern post-Pleistocene, interglacial climate. Today there are feral horses still living in those same environments. They find a sufficient mix of food to avoid toxins, they extract enough nutrition from forage to reproduce effectively and the timing of their gestation is not an issue. Of course, this criticism ignores the obvious fact that present-day horses are not competing for resources with ground sloths, mammoths, mastodons, camels, llamas, and bison. Similarly, mammoths survived the Pleistocene Holocene transition on isolated, uninhabited islands in the Mediterranean Sea[290] and on Wrangel Island in the Siberian Arctic[291] until 4,000 to 7,000 years ago.
- Large mammals should have been able to migrate, permanently or seasonally, if they found the temperature too extreme, the breeding season too short, or the rainfall too sparse or unpredictable.[292] Seasons vary geographically. By migrating away from the equator, herbivores could have found areas with growing seasons more favorable for finding food and breeding successfully. Modern-day African elephants migrate during periods of drought to places where there is apt to be water.[293]
- Large animals store more fat in their bodies than do medium-sized animals[294] and this should have allowed them to compensate for extreme seasonal fluctuations in food availability.
The extinction of the megafauna could have caused the disappearance of the mammoth steppe. Alaska now has low nutrient soil unable to support bison, mammoths, and horses. R. Dale Guthrie has claimed this as a cause of the extinction of the megafauna there; however, he may be interpreting it backwards. The loss of large herbivores to break up the permafrost allows the cold soils that are unable to support large herbivores today. Today, in the arctic, where trucks have broken the permafrost grasses and diverse flora and fauna can be supported.[295][296] In addition, Chapin (Chapin 1980) showed that simply adding fertilizer to the soil in Alaska could make grasses grow again like they did in the era of the mammoth steppe. Possibly, the extinction of the megafauna and the corresponding loss of dung is what led to low nutrient levels in modern-day soil and therefore is why the landscape can no longer support megafauna.
Arguments against both climate change and overkill
It may be observed that neither the overkill nor the climate change hypotheses can fully explain events: browsers, mixed feeders and non-ruminant grazer species suffered most, while relatively more ruminant grazers survived.[297] However, a broader variation of the overkill hypothesis may predict this, because changes in vegetation wrought by either Second Order Predation (see below)[298][299] or anthropogenic fire preferentially selects against browse species.[citation needed]
Hyperdisease hypothesis
Theory
The hyperdisease hypothesis, as advanced by Ross D. E. MacFee and Preston A. Marx, attributes the extinction of large mammals during the late Pleistocene to indirect effects of the newly arrived aboriginal humans.[300][301][302] The hyperdisease hypothesis proposes that humans or animals traveling with them (e.g., chickens or domestic dogs) introduced one or more highly virulent diseases into vulnerable populations of native mammals, eventually causing extinctions. The extinction was biased toward larger-sized species because smaller species have greater resilience because of their life history traits (e.g., shorter gestation time, greater population sizes, etc.). Humans are thought to be the cause because other earlier immigrations of mammals into North America from Eurasia did not cause extinctions.[300]
Diseases imported by people have been responsible for extinctions in the recent past; for example, bringing avian malaria to Hawaii has had a major impact on the isolated birds of the island.
If a disease was indeed responsible for the end-Pleistocene extinctions, then there are several criteria it must satisfy (see Table 7.3 in MacPhee & Marx 1997). First, the pathogen must have a stable carrier state in a reservoir species. That is, it must be able to sustain itself in the environment when there are no susceptible hosts available to infect. Second, the pathogen must have a high infection rate, such that it is able to infect virtually all individuals of all ages and sexes encountered. Third, it must be extremely lethal, with a mortality rate of c. 50–75%. Finally, it must have the ability to infect multiple host species without posing a serious threat to humans. Humans may be infected, but the disease must not be highly lethal or able to cause an epidemic.[citation needed]
One suggestion is that pathogens were transmitted by the expanding humans via the domesticated dogs they brought with them,[303] though this does not fit the timeline of extinctions in the Americas and Australia in particular.
Arguments against the hyperdisease hypothesis
- Generally speaking, disease has to be very virulent to kill off all the individuals in a genus or species. Even such a virulent disease as West Nile fever is unlikely to have caused extinction.[304]
- The disease would need to be implausibly selective while being simultaneously implausibly broad. Such a disease needs to be capable of killing off wolves such as Canis dirus or goats such as Oreamnos harringtoni while leaving other very similar species (Canis lupus and Oreamnos americanus, respectively) unaffected. It would need to be capable of killing off flightless birds while leaving closely related flighted species unaffected. Yet while remaining sufficiently selective to afflict only individual species within genera it must be capable of fatally infecting across such clades as birds, marsupials, placentals, testudines, and crocodilians. No disease with such a broad scope of fatal infectivity is known, much less one that remains simultaneously incapable of infecting numerous closely related species within those disparate clades. On the other hand, this objection does not account for the possibility of a variety of different diseases being introduced around the same era.[citation needed]
- Numerous species including wolves, mammoths, camelids, and horses had emigrated continually between Asia and North America over the past 100,000 years. For the disease hypothesis to be applicable there it would require that the population remain immunologically naive despite this constant transmission of genetic and pathogenic material.[citation needed]
- The dog-specific hypothesis cannot account for several major extinction events, notably the Americas (for reasons already covered) and Australia. Dogs did not arrive in Australia until approximately 35,000 years after the first humans arrived there, and approximately 30,000 years after the Australian megafaunal extinction was complete.[citation needed]
Second-order predation hypothesis
Scenario
The Second-Order Predation Hypothesis says that as humans entered the New World they continued their policy of killing predators, which had been successful in the Old World but because they were more efficient and because the fauna, both herbivores and carnivores, were more naive, they killed off enough carnivores to upset the ecological balance of the continent, causing overpopulation, environmental exhaustion, and environmental collapse. The hypothesis accounts for changes in animal, plant, and human populations.
The scenario is as follows:
- After the arrival of H. sapiens in the New World, existing predators must share the prey populations with this new predator. Because of this competition, populations of original, or first-order, predators cannot find enough food; they are in direct competition with humans.
- Second-order predation begins as humans begin to kill predators.
- Prey populations are no longer well controlled by predation. Killing of nonhuman predators by H. sapiens reduces their numbers to a point where these predators no longer regulate the size of the prey populations.
- Lack of regulation by first-order predators triggers boom-and-bust cycles in prey populations. Prey populations expand and consequently overgraze and over-browse the land. Soon the environment is no longer able to support them. As a result, many herbivores starve. Species that rely on the slowest recruiting food become extinct, followed by species that cannot extract the maximum benefit from every bit of their food.
- Boom-bust cycles in herbivore populations change the nature of the vegetative environment, with consequent climatic impacts on relative humidity and continentality. Through overgrazing and overbrowsing, mixed parkland becomes grassland, and climatic continentality increases.
Support
This has been supported by a computer model, the Pleistocene extinction model (PEM), which, using the same assumptions and values for all variables (herbivore population, herbivore recruitment rates, food needed per human, herbivore hunting rates, etc.) other than those for hunting of predators. It compares the overkill hypothesis (predator hunting = 0) with second-order predation (predator hunting varied between 0.01 and 0.05 for different runs). The findings are that second-order predation is more consistent with extinction than is overkill[305][306] (results graph at left).
The Pleistocene extinction model is the only test of multiple hypotheses and is the only model to specifically test combination hypotheses by artificially introducing sufficient climate change to cause extinction. When overkill and climate change are combined they balance each other out. Climate change reduces the number of plants, overkill removes animals, therefore fewer plants are eaten. Second-order predation combined with climate change exacerbates the effect of climate change.[298] (results graph at right).
The second-order predation hypothesis is supported by the observation above that there was a massive increase in bison populations.[307]
Second-order predation and other theories
- Climate change: Second-order predation accounts for the changes in vegetation, which in turn may account for the increase in continentality. Since the extinction is due to destruction of habitat it accounts for the loss of animals not hunted by humans. Second-order predation accounts for the dwarfing of animals as well as extinctions since animals that could survive and reproduce on less food would be selectively favored.
- Hyperdisease: The reduction of carnivores could have been from distemper or other carnivore disease carried by domestic dogs.
- Overkill: The observation that extinctions follow the arrival of humans is consistent with the second-order predation hypothesis.
Arguments against the second-order predation hypothesis
- The model specifically assumes high extinction rates in grasslands, but most extinct species ranged across numerous vegetation zones. Historical population densities of ungulates were very high in the Great Plains; savanna environments support high ungulate diversity throughout Africa, and extinction intensity was equally severe in forested environments.
- It is unable to explain why large herbivore populations were not regulated by surviving carnivores such as grizzly bears, wolves, pumas, and jaguars whose populations would have increased rapidly in response to the loss of competitors.
- It does not explain why almost all extinct carnivores were large herbivore specialists such as sabre toothed cats and short faced bears, but most hypocarnivores and generalized carnivores survived.
- There is no historical evidence of boom and bust cycles causing even local extinctions in regions where large mammal predators have been driven extinct by hunting. The recent hunting out of remaining predators throughout most of the United States has not caused massive vegetational change or dramatic boom and bust cycles in ungulates.
- It is not spatially explicit and does not track predator and prey species separately, whereas the multispecies overkill model does both.
- The multispecies model produces a mass extinction through indirect competition between herbivore species: small species with high reproductive rates subsidize predation on large species with low reproductive rates.[250] All prey species are lumped in the Pleistocene extinction model.
- Everything explained by the Pleistocene extinction model also is explained by the multispecies model, but with fewer assumptions, so the Pleistocene extinction model appears less parsimonious. However, the multispecies model does not explain shifts in vegetation, nor is it able to simulate alternative hypotheses. The multispecies model therefore necessitates additional assumptions and hence is less parsimonious.
Arguments against the second-order predation plus climate hypothesis
- It assumes decreases in vegetation due to climate change, but deglaciation doubled the habitable area of North America.
- Any vegetational changes that did occur failed to cause almost any extinctions of small vertebrates, and they are more narrowly distributed on average.
Younger Dryas impact hypothesis
First publicly presented at the Spring 2007 joint assembly of the American Geophysical Union in Acapulco, Mexico, the Younger Dryas impact hypothesis suggests that the mass extinction was caused by fragments of a disintegrating asteroid or comet 12,900 years ago. Using photomicrograph analysis, research published in January 2009 has found evidence of nanodiamonds in the soil from six sites across North America including Arizona, Minnesota, Oklahoma, South Carolina and two Canadian sites. Similar research found nanodiamonds in the Greenland ice sheet.[308][309][310]
Arguments against/for the impact hypothesis
Debate around this hypothesis has included, among other things, the lack of an impact crater, relatively small increased level of iridium in the soil, and the relative probability of such an event. That said, it took 10 years after publication of the Alvarez theory before scientists found the Chicxulub crater. If the bolide struck the Laurentide ice sheet as hypothesized by Firestone et al. (2007), a typical impact crater would not be visible.
A spike in platinum was found in the Greenland ice cores by Petaev et al. (2013), which they view as a global signal.[311] Confirmation came in 2017 with the report that the Pt spike had been found at "11 widely separated archaeological bulk sedimentary sequences."[312] Wolbach et al. reported in 2018 that "YDB peaks in Pt were observed at 28 sites" in total, including the 11 reported earlier and the one from Greenland.[313]
- Some have reported a lack of evidence for a population decline among the Paleoindians at 12,900 ± 100 calBP.[314][315][316] However, others have reported finding such evidence.[317]
- There is evidence that the megafaunal extinctions that occurred across northern Eurasia, North America and South America at the end of the Pleistocene were not synchronous as the bolide theory would predict. The extinctions in South America appear to have occurred at least 400 years after those in North America.[318][319][320]
- Additionally, some island megafaunal populations survived thousands of years longer than populations of the same or related species on nearby continents; examples include the survival of woolly mammoths on Wrangel Island until 3700 BP,[318][319] and the survival of ground sloths in the Antilles until 4700 cal BP.[318][319][320]
- Several markers for the proposed impact event are disputed. Opponents have asserted that the carbon spherules originated as fungal structures and/or insect fecal pellets,[321] and that the claimed nanodiamonds are actually misidentified graphene and graphene/graphane oxide aggregates.[322][323] An analysis of a similar Younger Dryas boundary layer in Belgium also did not show evidence of a bolide impact.[324]
- However, proponents of the hypothesis have responded to defend their results, disputing the accusation of irreproducibility and/or replicating their findings.[325][326][327][328][329][330] Prior to finding of a widespread Pt spike on the continents, Pleistocene expert Wallace Broecker had already changed his mind about the YDIH: "The Greenland platinum peak makes clear that an extraterrestrial impact occurred close to the onset of the YD."[331]
See also
- Earth:Holocene extinction – Ongoing extinction event caused by human activity
- Biology:List of quaternary mammalian fauna of China
- Biology:Megafauna – Large animals
- Biology:Pleistocene megafauna – Large animals that lived during the Pleistocene
- Biology:Pleistocene rewilding – Ecological practice
- Earth:Toba catastrophe theory – Supereruption 74,000 years ago that may have caused a global volcanic winter
References
- ↑ Martin, Paul S.; Klein, Richard G. (1989-01-01) (in en). Quaternary Extinctions: A Prehistoric Revolution. University of Arizona Press. ISBN 978-0-8165-1100-6. https://books.google.com/books?id=qIDC7ybvHQEC.
- ↑ 2.0 2.1 Smith, Felisa A. (April 20, 2018). "Body size downgrading of mammals over the late Quaternary". Science 360 (6386): 310–313. doi:10.1126/science.aao5987. PMID 29674591.
- ↑ Kolbert, Elizabeth (2014). The Sixth Extinction: An Unnatural History. Bloomsbury Publishing. ISBN 9780805092998.
- ↑ Koch, Paul L.; Barnosky, Anthony D. (2006-01-01). "Late Quaternary Extinctions: State of the Debate". Annual Review of Ecology, Evolution, and Systematics 37 (1): 215–250. doi:10.1146/annurev.ecolsys.34.011802.132415. https://semanticscholar.org/paper/2c670ea1d5327e9c0e76e976102c2ba54e1a083e.
- ↑ Stringer, Chris; Galway-Witham, Julia (2017). "Palaeoanthropology: On the origin of our species". Nature 546 (7657): 212–214. doi:10.1038/546212a. PMID 28593955. Bibcode: 2017Natur.546..212S.
- ↑ 6.0 6.1 Callaway, Ewen (2015). "Teeth from China reveal early human trek out of Africa". Nature. doi:10.1038/nature.2015.18566. http://www.nature.com/news/teeth-from-china-reveal-early-human-trek-out-of-africa-1.18566.
- ↑ 7.0 7.1 Marwick, Ben. "Buried tools and pigments tell a new history of humans in Australia for 65,000 years". The Conversation. https://theconversation.com/buried-tools-and-pigments-tell-a-new-history-of-humans-in-australia-for-65-000-years-81021.
- ↑ 8.0 8.1 Bourgeon, Lauriane; Burke, Ariane; Higham, Thomas (2017-01-06). "Earliest Human Presence in North America Dated to the Last Glacial Maximum: New Radiocarbon Dates from Bluefish Caves, Canada". PLOS ONE 12 (1): e0169486. doi:10.1371/journal.pone.0169486. ISSN 1932-6203. PMID 28060931. Bibcode: 2017PLoSO..1269486B.
- ↑ Curry, Andrew (2012-05-03). "Ancient migration: Coming to America". Nature 485 (7396): 30–32. doi:10.1038/485030a. PMID 22552076. Bibcode: 2012Natur.485...30C.
- ↑ "Humans didn't wait on melting ice to settle the Americas". Science | AAAS. 2016-06-06. http://www.sciencemag.org/news/2016/06/humans-didn-t-wait-melting-ice-settle-americas.
- ↑ Callaway, Ewen (2017-09-07). "Skeleton plundered from Mexican cave was one of the Americas' oldest". Nature 549 (7670): 14–15. doi:10.1038/nature.2017.22521. PMID 28880302. Bibcode: 2017Natur.549...14C.
- ↑ Sandom, Christopher; Faurby, Søren; Sandel, Brody; Svenning, Jens-Christian (4 June 2014). "Global late Quaternary megafauna extinctions linked to humans, not climate change". Proceedings of the Royal Society B 281 (1787): 20133254. doi:10.1098/rspb.2013.3254. PMID 24898370.
- ↑ Extinctions in Near Time. Pages 46–47. By R. D. E. MacPhee. Springer Press. 1999.
- ↑ Vignieri, S. (25 July 2014). "Vanishing fauna (Special issue)". Science 345 (6195): 392–412. doi:10.1126/science.345.6195.392. PMID 25061199. Bibcode: 2014Sci...345..392V. "Although some debate persists, most of the evidence suggests that humans were responsible for extinction of this Pleistocene fauna, and we continue to drive animal extinctions today through the destruction of wild lands, consumption of animals as a resource or a luxury, and persecution of species we see as threats or competitors.".
- ↑ Faurby, Søren; Svenning, Jens-Christian (2015). "Historic and prehistoric human‐driven extinctions have reshaped global mammal diversity patterns". Diversity and Distributions 21 (10): 1155–1166. doi:10.1111/ddi.12369.
- ↑ Putshkov, P. V. (1997). "Were the Mammoths killed by the warming? (Testing of the climatic versions of the Wurm extinctions)". Vestnik Zoologii Supplement No.4.
- ↑ Rabanus-Wallace, M. Timothy; Wooller, Matthew J.; Zazula, Grant D.; Shute, Elen; Jahren, A. Hope; Kosintsev, Pavel; Burns, James A.; Breen, James et al. (2017). "Megafaunal isotopes reveal role of increased moisture on rangeland during late Pleistocene extinctions". Nature Ecology & Evolution 1 (5): 0125. doi:10.1038/s41559-017-0125. PMID 28812683.
- ↑ 18.0 18.1 Gillespie, Richard (2008). "Updating Martin's global extinction model". Quaternary Science Reviews 27 (27–28): 2522–2529. doi:10.1016/j.quascirev.2008.09.007. Bibcode: 2008QSRv...27.2522G.
- ↑ Grayson, Donald K.; Meltzer, David J. (2002). "Clovis Hunting and Large Mammal Extinction: A Critical Review of the Evidence". Journal of World Prehistory 16 (4): 313–359. doi:10.1023/A:1022912030020.
- ↑ Anderson, Paul K. (July 1995). "Competition, Predation, and the Evolution and Extinction of Steller's Sea Cow, Hydrodamalis Gigas". Marine Mammal Science 11 (3): 391–4. doi:10.1111/j.1748-7692.1995.tb00294.x.
- ↑ Lan, Tianying; Lindqvist, Charlotte (2018). "Paleogenomics: Genome-Scale Analysis of Ancient DNA and Population and Evolutionary Genomic Inferences". in Lindqvist, C.; Rajora, O.. Population Genomics. pp. 323–360. doi:10.1007/13836_2017_7. ISBN 978-3-030-04587-6.
- ↑ Werdelin, Lars; Lewis, Margaret E. (2013-03-06). "Temporal Change in Functional Richness and Evenness in the Eastern African Plio-Pleistocene Carnivoran Guild". PLOS ONE 8 (3): e57944. doi:10.1371/journal.pone.0057944. ISSN 1932-6203. PMID 23483948. Bibcode: 2013PLoSO...857944W.
- ↑ Tollefson, Jeff (2012). "Early humans linked to large-carnivore extinctions". Nature. doi:10.1038/nature.2012.10508. http://www.nature.com/news/early-humans-linked-to-large-carnivore-extinctions-1.10508?nc=1370632369631.
- ↑ Wong, Kate. "Rise of Humans 2 Million Years Ago Doomed Large Carnivores". Scientific American Blog Network. https://blogs.scientificamerican.com/observations/rise-of-humans-two-million-years-ago-doomed-large-carnivores/.
- ↑ Gunz, Philipp; Harvati, Katerina; Benazzi, Stefano; Cabec, Adeline Le; Bergmann, Inga; Skinner, Matthew M.; Neubauer, Simon; Freidline, Sarah E. et al. (June 2017). "New fossils from Jebel Irhoud, Morocco and the pan-African origin of Homo sapiens". Nature 546 (7657): 289–292. doi:10.1038/nature22336. ISSN 1476-4687. PMID 28593953. Bibcode: 2017Natur.546..289H. https://kar.kent.ac.uk/62267/1/Submission_288356_1_art_file_2637492_j96j1b.pdf.
- ↑ Hublin, Jean-Jacques; Ben-Ncer, Abdelouahed; Bailey, Shara E.; Freidline, Sarah E.; Neubauer, Simon; Skinner, Matthew M.; Bergmann, Inga; Le Cabec, Adeline et al. (2017-06-07). "New fossils from Jebel Irhoud, Morocco and the pan-African origin of Homo sapiens". Nature 546 (7657): 289–292. doi:10.1038/nature22336. ISSN 0028-0836. PMID 28593953. Bibcode: 2017Natur.546..289H. https://kar.kent.ac.uk/62267/1/Submission_288356_1_art_file_2637492_j96j1b.pdf.
- ↑ "These Early Humans Lived 300,000 Years Ago—But Had Modern Faces". 2017-06-07. http://news.nationalgeographic.com/2017/06/morocco-early-human-fossils-anthropology-science/.
- ↑ Akey, Joshua M.; Wolf, Aaron B. (2018-05-31). "Outstanding questions in the study of archaic hominin admixture". PLOS Genetics 14 (5): e1007349. doi:10.1371/journal.pgen.1007349. ISSN 1553-7404. PMID 29852022.
- ↑ Chan, Paul K. S.; Burk, Robert D.; DeSalle, Rob; Chan, Martin C. W.; Law, Priscilla T. Y.; Boon, Siaw Shi; Ho, Wendy C. S.; Chen, Zigui (2017-11-01). "Ancient Evolution and Dispersion of Human Papillomavirus 58 Variants". Journal of Virology 91 (21): e01285–17. doi:10.1128/JVI.01285-17. ISSN 0022-538X. PMID 28794033.
- ↑ Gokcumen, Omer; Ruhl, Stefan; Blekhman, Ran; DeGiorgio, Michael; Flanagan, Colin; Alachiotis, Nikolaos; Taskent, Recep Ozgur; Pavlidis, Pavlos et al. (2017-10-01). "Archaic Hominin Introgression in Africa Contributes to Functional Salivary MUC7 Genetic Variation". Molecular Biology and Evolution 34 (10): 2704–2715. doi:10.1093/molbev/msx206. ISSN 0737-4038. PMID 28957509.
- ↑ Callaway, Ewen (2016). "Evidence mounts for interbreeding bonanza in ancient human species". Nature News. doi:10.1038/nature.2016.19394. http://www.nature.com/news/evidence-mounts-for-interbreeding-bonanza-in-ancient-human-species-1.19394.
- ↑ Callaway, Ewen (2013). "Mystery humans spiced up ancients' sex lives". Nature News. doi:10.1038/nature.2013.14196. http://www.nature.com/news/mystery-humans-spiced-up-ancients-sex-lives-1.14196.
- ↑ Lachance, Joseph; Vernot, Benjamin; Elbers, Clara C.; Ferwerda, Bart; Froment, Alain; Bodo, Jean-Marie; Lema, Godfrey; Fu, Wenqing et al. (2012-08-03). "Evolutionary history and adaptation from high-coverage whole-genome sequences of diverse African hunter-gatherers". Cell 150 (3): 457–469. doi:10.1016/j.cell.2012.07.009. ISSN 0092-8674. PMID 22840920.
- ↑ Hsieh, PingHsun; Woerner, August E.; Wall, Jeffrey D.; Lachance, Joseph; Tishkoff, Sarah A.; Gutenkunst, Ryan N.; Hammer, Michael F. (March 2016). "Model-based analyses of whole-genome data reveal a complex evolutionary history involving archaic introgression in Central African Pygmies". Genome Research 26 (3): 291–300. doi:10.1101/gr.196634.115. ISSN 1549-5469. PMID 26888264.
- ↑ Callaway, Ewen (2012). "Hunter-gatherer genomes a trove of genetic diversity". Nature News. doi:10.1038/nature.2012.11076. http://www.nature.com/news/hunter-gatherer-genomes-a-trove-of-genetic-diversity-1.11076.
- ↑ Posth, Cosimo; Wißing, Christoph; Kitagawa, Keiko; Pagani, Luca; Holstein, Laura van; Racimo, Fernando; Wehrberger, Kurt; Conard, Nicholas J. et al. (2017-07-04). "Deeply divergent archaic mitochondrial genome provides lower time boundary for African gene flow into Neanderthals". Nature Communications 8: ncomms16046. doi:10.1038/ncomms16046. PMID 28675384. Bibcode: 2017NatCo...816046P.
- ↑ Zimmer, Carl (2017-07-04). "In Neanderthal DNA, Signs of a Mysterious Human Migration". The New York Times. ISSN 0362-4331. https://www.nytimes.com/2017/07/04/science/neanderthals-dna-homo-sapiens-human-evolution.html.
- ↑ Castellano, Sergi; Siepel, Adam; Meyer, Matthias; Pääbo, Svante; Viola, Bence; Andrés, Aida M.; Marques-Bonet, Tomas; Gušic, Ivan et al. (February 2016). "Ancient gene flow from early modern humans into Eastern Neanderthals". Nature 530 (7591): 429–433. doi:10.1038/nature16544. ISSN 1476-4687. PMID 26886800. Bibcode: 2016Natur.530..429K.
- ↑ "When Neanderthals Replaced Us | DiscoverMagazine.com". Discover Magazine. http://discovermagazine.com/2016/june/15-when-neanderthals-replaced-us.
- ↑ Lawler, Andrew (2011-01-28). "Did Modern Humans Travel Out of Africa Via Arabia?". Science 331 (6016): 387. doi:10.1126/science.331.6016.387. ISSN 0036-8075. PMID 21273459. Bibcode: 2011Sci...331..387L.
- ↑ "The First Humans Moved From Africa To China -- Not Europe". Gizmodo Australia. 2015-10-16. https://www.gizmodo.com.au/2015/10/the-first-humans-moved-from-africa-to-china-not-europe/.
- ↑ Westaway, K. E.; Louys, J.; Awe, R. Due; Morwood, M. J.; Price, G. J.; Zhao, J.-x; Aubert, M.; Joannes-Boyau, R. et al. (2017-08-17). "An early modern human presence in Sumatra 73,000–63,000 years ago". Nature 548 (7667): 322–325. doi:10.1038/nature23452. ISSN 0028-0836. PMID 28792933. Bibcode: 2017Natur.548..322W. https://eprints.soton.ac.uk/412591/1/combinbed_files.pdf.
- ↑ "Were Modern Humans in Indonesia 73,000 Years Ago? - Dead Things". Dead Things. 2017-08-09. http://blogs.discovermagazine.com/deadthings/2017/08/09/were-modern-humans-in-indonesia-73000-years-ago/#.WbYZQdN97Vo.
- ↑ Pagani, Luca; Lawson, Daniel John; Jagoda, Evelyn; Mörseburg, Alexander; Eriksson, Anders; Mitt, Mario; Clemente, Florian; Hudjashov, Georgi et al. (2016-09-21). "Genomic analyses inform on migration events during the peopling of Eurasia". Nature 538 (7624): 238–242. doi:10.1038/nature19792. ISSN 1476-4687. PMID 27654910. Bibcode: 2016Natur.538..238P.
- ↑ "Almost all living people outside of Africa trace back to a single migration more than 50,000 years ago". Science | AAAS. 2016-09-20. http://www.sciencemag.org/news/2016/09/almost-all-living-people-outside-africa-trace-back-single-migration-more-50000-years.
- ↑ "Human Migration: Challenging the Chronology of Our First Road Trip - Dead Things". Dead Things. 2016-09-21. http://blogs.discovermagazine.com/deadthings/2016/09/21/human-migration-challenging-the-chronology-of-our-first-road-trip/#.Wd9iqtOg_Vr.
- ↑ Wu, Xiaohong; Zhang, Chi; Goldberg, Paul; Cohen, David; Pan, Yan; Arpin, Trina; Bar-Yosef, Ofer (2012-06-29). "Early Pottery at 20,000 Years Ago in Xianrendong Cave, China". Science 336 (6089): 1696–1700. doi:10.1126/science.1218643. ISSN 0036-8075. PMID 22745428. Bibcode: 2012Sci...336.1696W. https://semanticscholar.org/paper/b6ccd811c8311dc706fe38cc4508341da59646bf.
- ↑ Julien Louys; Darren Curnoe; Haowen Tong. (2007). "Characteristics of Pleistocene megafauna extinctions in Southeast Asia". Palaeogeography, Palaeoclimatology, Palaeoecology 243 (1–2): 152–173. doi:10.1016/j.palaeo.2006.07.011.
- ↑ HASEGAWA Y.,OKUMURA Y., TATSUKAWA H. (2009). "First record of Late Pleistocene Bison from the fissure deposits of the Kuzuu Limestone, Yamasuge,Sano-shi,Tochigi Prefecture, Japan". Gunma Museum of Natural History and Kuzuu Fossil Museum 13: 47–52. http://www.gmnh.pref.gunma.jp/wp-content/uploads/bulletin13_5.pdf.
- ↑ Kurosawa Y.. "モノが語る牛と人間の文化 – ② 岩手の牛たち". LIAJ News No.109- Oshu City Cattle Museum p. 29-31. http://liaj.lin.gr.jp/japanese/liajnews/liaj10909.pdf.
- ↑ Bison hanaizumiensis - TrekGEO
- ↑ Rozzi, Roberto (2017-02-01). "A new extinct dwarfed buffalo from Sulawesi and the evolution of the subgenus Anoa: An interdisciplinary perspective". Quaternary Science Reviews 157: 188–205. doi:10.1016/j.quascirev.2016.12.011. Bibcode: 2017QSRv..157..188R.
- ↑ "Fossilworks: Megalovis". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=42796.
- ↑ Clark, J. Desmond (1982-02-25). The Cambridge History of Africa. Cambridge University Press. ISBN 9780521222150. https://books.google.com/books?id=Y8iiqIZRhQoC.
- ↑ 55.0 55.1 "Fossilworks: Gazella". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=42774.
- ↑ Geist, Valerius (1998-01-01). Deer of the World: Their Evolution, Behaviour, and Ecology. Stackpole Books. ISBN 9780811704960. https://books.google.com/books?id=bcWZX-IMEVkC&q=sinomegaceros&pg=PA125.
- ↑ "Fossilworks: Dorcabune". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=42599.
- ↑ Horwitz, Liora Kolska; Tchernov, Eitan (1990-01-01). "Cultural and Environmental Implications of Hippopotamus Bone Remains in Archaeological Contexts in the Levant". Bulletin of the American Schools of Oriental Research 280 (280): 67–76. doi:10.2307/1357310.
- ↑ Haas, Georg (1953-01-01). "On the Occurrence of Hippopotamus in the Iron Age of the Coastal Area of Israel (Tell Qasîleh)". Bulletin of the American Schools of Oriental Research 132 (132): 30–34. doi:10.2307/1355798.
- ↑ Jukar, Advait M.; Patnaik, Rajeev; Chauhan, Parth R.; Li, Hong-Chun; Lin, Jih-Pai (2019-09-10). "The youngest occurrence of Hexaprotodon Falconer and Cautley, 1836 (Hippopotamidae, Mammalia) from South Asia with a discussion on its extinction" (in en). Quaternary International. AMS 14C Applications II 528: 130–137. doi:10.1016/j.quaint.2019.01.005. ISSN 1040-6182. https://www.sciencedirect.com/science/article/pii/S1040618218310085.
- ↑ Heinrich, Earl (31 October 2013). "Ancient Nubia". Cambridge Online Histories. http://www.earlheinrich.com/Ancient%20Nubia/_Private/Ch%203%20excerpt.pdf.
- ↑ "Fossilworks: Rhinoceros philippinensis". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=164277.
- ↑ Ohdachi, Satoshi D.; Ishibashi, Yasuyuki; Iwasa, Masahiro A.; Fukui, Dai; Saitoh, Takashi (2015). The wild mammals of Japan (2nd ed.). Shoukadoh. ISBN 9784879746917. OCLC 946607025.
- ↑ "The Last Wild Tigers". Audubon. 2014-06-25. http://www.audubon.org/news/the-last-wild-tigers.
- ↑ "PBDB". http://www.paleobiodb.org/classic/displayReference?reference_no=34644.
- ↑ "Rock paintings show species that roamed India". http://www.newindianexpress.com/nation/article76865.ece?service=print.
- ↑ Feldhamer, George A.; Drickamer, Lee C.; Vessey, Stephen H.; Merritt, Joseph F.; Krajewski, Carey (2015-01-01). Mammalogy: Adaptation, Diversity, Ecology. JHU Press. ISBN 9781421415888. https://books.google.com/books?id=Ugq5BgAAQBAJ&q=aardvark+indian+fossils&pg=PA349.
- ↑ Larramendi, Asier (2015). "Shoulder height, body mass and shape of proboscideans". Acta Palaeontologica Polonica. doi:10.4202/app.00136.2014. http://www.app.pan.pl/archive/published/app60/app001362014_acc.pdf.
- ↑ Pushinka, Diana (2007). "The Pleistocene easternmost distribution in Eurasia of the species associated with the Eemian Paleoloxodon antiquus assemblage". Mammal Review 37 (3): 224–245. doi:10.1111/j.1365-2907.2007.00109.x. http://www.rhinoresourcecenter.com/pdf_files/131/1318084071.pdf.
- ↑ Watanabe, Junya; Matsuoka, Hiroshige (2015-11-02). "Flightless diving duck (Aves, Anatidae) from the Pleistocene of Shiriya, northeast Japan". Journal of Vertebrate Paleontology 35 (6): e994745. doi:10.1080/02724634.2014.994745.
- ↑ Hansford, James P.; Turvey, Samuel T. (2018-09-26). "Unexpected diversity within the extinct elephant birds (Aves: Aepyornithidae) and a new identity for the world's largest bird". R Soc Open Sci. 5,9 (181295): 181295. doi:10.1098/rsos.181295. PMID 30839722. Bibcode: 2018RSOS....581295H.
- ↑ 72.0 72.1 Callaway, Ewen (2016-03-17). "Oldest ancient-human DNA details dawn of Neanderthals". Nature News 531 (7594): 296–286. doi:10.1038/531286a. PMID 26983523. Bibcode: 2016Natur.531..296C.
- ↑ Lao, Oscar; Bertranpetit, Jaume; Mondal, Mayukh (2019-01-16). "Approximate Bayesian computation with deep learning supports a third archaic introgression in Asia and Oceania". Nature Communications 10 (1): 246. doi:10.1038/s41467-018-08089-7. ISSN 2041-1723. PMID 30651539. Bibcode: 2019NatCo..10..246M.
- ↑ Pennisi, Elizabeth (2013-05-17). "More Genomes From Denisova Cave Show Mixing of Early Human Groups". Science 340 (6134): 799. doi:10.1126/science.340.6134.799. ISSN 0036-8075. PMID 23687020. Bibcode: 2013Sci...340..799P.
- ↑ Saey, Tina Hesman (2016-11-02). "DNA data offer evidence of unknown extinct human relative". https://www.sciencenews.org/article/dna-data-offer-evidence-unknown-extinct-human-relative.
- ↑ 76.0 76.1 Kahlke, Ralf-Dietrich (2014). "The origin of Eurasian Mammoth Faunas (Mammuthus-Coelodonta Faunal Complex)". Quaternary Science Reviews 96: 32–49. doi:10.1016/j.quascirev.2013.01.012. Bibcode: 2014QSRv...96...32K. http://www.rhinoresourcecenter.com/pdf_files/140/1404659577.pdf.
- ↑ 77.0 77.1 77.2 Zimov, S. A.; Zimov, N. S.; Tikhonov, A. N.; Chapin III, F. S. (2012-12-04). "Mammoth steppe: a high-productivity phenomenon". Quaternary Science Reviews 57: 26–45. doi:10.1016/j.quascirev.2012.10.005. Bibcode: 2012QSRv...57...26Z.
- ↑ Sher, A. V.; Kuzmina, S. A.; Kuznetsova, T. V.; Sulerzhitsky, L. D. (2005-03-01). "New insights into the Weichselian environment and climate of the East Siberian Arctic, derived from fossil insects, plants, and mammals". Quaternary Science Reviews 24 (5–6): 533–569. doi:10.1016/j.quascirev.2004.09.007. Bibcode: 2005QSRv...24..533S. http://oceanrep.geomar.de/28296/1/2005_Sher-etal-New_QSR-25.pdf.
- ↑ Adams, J. M.; Faure, H.; Faure-Denard, L.; McGlade, J. M.; Woodward, F. I. (1990-12-27). "Increases in terrestrial carbon storage from the Last Glacial Maximum to the present". Nature 348 (6303): 711–714. doi:10.1038/348711a0. Bibcode: 1990Natur.348..711A.
- ↑ Álvarez-Lao, Diego J.; García, Nuria (2011-03-15). "Geographical distribution of Pleistocene cold-adapted large mammal faunas in the Iberian Peninsula". Quaternary International. Quaternary Floral and Faunal Assemblages: Ecological and Taphonomical Investigations 233 (2): 159–170. doi:10.1016/j.quaint.2010.04.017. Bibcode: 2011QuInt.233..159A.
- ↑ 81.0 81.1 Hoffecker, John F.; Elias, Scott A. (2012-05-29). Human Ecology of Beringia. Columbia University Press. ISBN 9780231503884. https://books.google.com/books?id=DQkpVuA7_6UC&q=baikal+yak&pg=PA56.
- ↑ 82.0 82.1 82.2 82.3 82.4 Vereshchagin, N. K.; Baryshnikov, G. F. (1991-01-01). "The ecological structure of the "Mammoth Fauna" in Eurasia". Annales Zoologici Fennici 28 (3/4): 253–259.
- ↑ Martin, Paul S.; Klein, Richard G. (1989-01-01). Quaternary Extinctions: A Prehistoric Revolution. University of Arizona Press. ISBN 9780816511006. https://books.google.com/books?id=qIDC7ybvHQEC&q=parabubalis+capricornis&pg=PA489.
- ↑ Naish, Darren. "The remarkable life appearance of the Woolly rhino". Scientific American Blog Network. https://blogs.scientificamerican.com/tetrapod-zoology/the-remarkable-life-appearance-of-the-woolly-rhino/.
- ↑ Diego J. ÁLVAREZ-LAO. "New discoveries of woolly mammoth and woolly rhinoceros from northern Iberia". Greece VIth International Conference on Mammoths and their Relatives, Special Volume 102. http://www.rhinoresourcecenter.com/index.php?s=1&act=pdfviewer&id=1402660443&folder=140.
- ↑ Pushinka, Diana (2007). "The Pleistocene easternmost distribution in Eurasia of the species associated with the Eemian Paleoloxodon antiquus assemblage". Mammal Review 37 (3): 224–245. doi:10.1111/j.1365-2907.2007.00109.x. http://www.rhinoresourcecenter.com/pdf_files/131/1318084071.pdf.
- ↑ Anthony J. Stuart; Adrian M. LiSter. "Patterns of Late Quaternary megafaunal extinctions in Europe and northern Asia". CFS Courier Forschungsinstitut Senckenberg 259: 289–299. https://www.researchgate.net/publication/264785182. Retrieved 2017-03-28.
- ↑ Fuss, Jochen; Spassov, Nikolai; Begun, David R.; Böhme, Madelaine (2017-05-22). "Potential hominin affinities of Graecopithecus from the Late Miocene of Europe". PLOS ONE 12 (5): e0177127. doi:10.1371/journal.pone.0177127. ISSN 1932-6203. PMID 28531170. Bibcode: 2017PLoSO..1277127F.
- ↑ Gierliński, Gerard D.; Niedźwiedzki, Grzegorz; Lockley, Martin G.; Athanassiou, Athanassios; Fassoulas, Charalampos; Dubicka, Zofia; Boczarowski, Andrzej; Bennett, Matthew R. et al. (2017). "Possible hominin footprints from the late Miocene (c. 5.7 Ma) of Crete?". Proceedings of the Geologists' Association 128 (5–6): 697–710. doi:10.1016/j.pgeola.2017.07.006.
- ↑ "What Made These Footprints 5.7 Million Years Ago? - Dead Things". Dead Things. 2017-09-01. http://blogs.discovermagazine.com/deadthings/2017/09/01/what-made-these-footprints-5-7-million-years-ago/#.WbYbeNN97Vp.
- ↑ Pääbo, Svante; Prüfer, Kay; Kelso, Janet; Viola, Bence; Carbonell, Eudald; Castro, José María Bermúdez de; Ana Gracia; Martínez, Ignacio et al. (March 2016). "Nuclear DNA sequences from the Middle Pleistocene Sima de los Huesos hominins". Nature 531 (7595): 504–507. doi:10.1038/nature17405. ISSN 1476-4687. PMID 26976447. Bibcode: 2016Natur.531..504M.
- ↑ See Figure 1 in L.A. Orlova, Y.V. Kuzmin, A.J. Stuart, A.N. Tikhonov (2001). "Chronology and environment of woolly mammoth (Mammuthus primigenius Blum.) extinction in northern Asia". The World of Elephants - International Congress, Rome 2001. https://fdocuments.net/document/mammuthus-primigenius-blumenbach-extinction-in-northern-asia.html. Retrieved 2017-04-07.
- ↑ Owen-Smith, R.N. (1992). Megaherbivores: The influence of very large body size on ecology. Cambridge studies in ecology. Cambridge: Cambridge Univ. Press. ISBN 978-0-521-42637-4.
- ↑ Whitney-Smith, E. (2006). Clovis and Extinctions – Overkill, Second Order Predation, Environmental Degradation in a Non-equilibrium Ecosystem "Clovis Age Continent". University of New Mexico Press.
- ↑ Marsolier-Kergoat, Marie-Claude; Palacio, Pauline; Berthonaud, Véronique; Maksud, Frédéric; Stafford, Thomas; Bégouën, Robert; Elalouf, Jean-Marc (2015-06-17). "Hunting the Extinct Steppe Bison (Bison priscus) Mitochondrial Genome in the Trois-Frères Paleolithic Painted Cave". PLOS ONE 10 (6): e0128267. doi:10.1371/journal.pone.0128267. PMID 26083419. Bibcode: 2015PLoSO..1028267M.
- ↑ Palacio, Pauline; Berthonaud, Véronique; Guérin, Claude; Lambourdière, Josie; Maksud, Frédéric; Philippe, Michel; Plaire, Delphine; Stafford, Thomas et al. (2017-01-01). "Genome data on the extinct Bison schoetensacki establish it as a sister species of the extant European bison (Bison bonasus)". BMC Evolutionary Biology 17 (1): 48. doi:10.1186/s12862-017-0894-2. PMID 28187706.
- ↑ 97.0 97.1 Rivals, Florent (2006). "Découverte de Capra caucasica et d'Hemitragus cedrensis (Mammalia, Bovidae) dans les niveaux du Pléistocène supérieur de la Caune de l'Arago (Tautavel, France) : Implication biochronologique dans le contexte du Bassin Méditerranéen". Geobios 39: 85–102. doi:10.1016/j.geobios.2004.08.004.
- ↑ 98.0 98.1 CRÉGUT-BONNOURE, Evelyne (12 March 2009). "Biochronologie et grands mammifères au Pléistocène moyen et supérieur en Europe occidentale : l'Apport des genres hemitragus et capra" (in fr). Quaternaire 20: 481–508. doi:10.4000/quaternaire.5345.
- ↑ (1924–1988)., Kurtén, Björn (2008-01-01). Pleistocene mammals of Europe. Aldine Transaction. ISBN 9780202309538. OCLC 751413776.
- ↑ Baryshnikov, G.; Tikhonov, A. (1994-10-01). "Notes on skulls of Pleistocene Saiga of Northern Eurasia". Historical Biology 8 (1–4): 209–234. doi:10.1080/10292389409380478.
- ↑ 101.0 101.1 101.2 101.3 Hoffecker, John F.; Elias, Scott A. (2012-05-29). Human Ecology of Beringia. Columbia University Press. ISBN 9780231503884. https://books.google.com/books?id=DQkpVuA7_6UC.
- ↑ Sanz, Montserrat; Daura, Joan; Brugal, Jean-Philip (2014-01-01). "First occurrence of the extinct deer Haploidoceros in the Iberian Peninsula in the Upper Pleistocene of the Cova del Rinoceront (Castelldefels, Barcelona)". Comptes Rendus Palevol 13 (1): 27–40. doi:10.1016/j.crpv.2013.06.005.
- ↑ Rivals, Florent; Sanz, Montserrat; Daura, Joan (2016-05-01). "First reconstruction of the dietary traits of the Mediterranean deer (Haploidoceros mediterraneus) from the Cova del Rinoceront (NE Iberian Peninsula)". Palaeogeography, Palaeoclimatology, Palaeoecology 449: 101–107. doi:10.1016/j.palaeo.2016.02.014. Bibcode: 2016PPP...449..101R.
- ↑ Abbazzi, Laura; Azzaroli, A. (1995-09-01). "Occurrence of palmated Cervus elaphus from Italian late Pleistocene localities". Rendiconti Lincei 6 (3): 189–206. doi:10.1007/BF03001667. ISSN 1120-6349.
- ↑ 105.0 105.1 Elias, Scott; Mock, Cary (2013-03-25). Encyclopedia of Quaternary Science. Newnes. ISBN 9780444536426. https://books.google.com/books?id=TSTnzG6yetsC.
- ↑ "Habitat conditions for Camelus knoblochi and factors in its extinction by Vadim V. Titov". http://www.paleorostov.narod.ru/Titov_Camelus_knoblochi.pdf.
- ↑ 107.0 107.1 107.2 Foronova, I. (2006). "Late quaternary equids (genus Equus) of South-western and South-central Siberia". in M. Mashkour. Equids in time and space. Papers in honour of Véra Eisenmann. Proceedings of the 9th conference of the International Council of Archaeozoology, Durham, August 2002. Oxbow Books. pp. 20–30.
- ↑ Chase, Philip G. (2009-01-01). The Cave of Fontéchevade: Recent Excavations and Their Paleoanthropological Implications. Cambridge University Press. ISBN 9780521898447. https://books.google.com/books?id=XTPbVI5Gf6MC&q=equus+gallicus&pg=PA146.
- ↑ Yanko-Hombach, Valentina; Gilbert, Allan S.; Panin, Nicolae; Dolukhanov, Pavel M. (2006-11-15). The Black Sea Flood Question: Changes in Coastline, Climate and Human Settlement. Springer Science & Business Media. ISBN 9781402053023. https://books.google.com/books?id=sDYXosqZpegC&q=equus+latipes&pg=PA288.
- ↑ Hopkins, David M.; Matthews, John V.; Schweger, Charles E. (2013-09-17). Paleoecology of Beringia. Elsevier. ISBN 9781483273402. https://books.google.com/books?id=ZxolBQAAQBAJ&q=equus+lenensis&pg=PA349.
- ↑ Lu, Dan; Yang, Yangheshan; Li, Qiang; Ni, Xijun (2021-07-30). "A late Pleistocene fossil from Northeastern China is the first record of the dire wolf (Carnivora: Canis dirus) in Eurasia" (in en). Quaternary International. Cave Deposits from Luotuo Hill, Northeast China: A Geochronologically Calibrated Mammalian Biostratigraphic Standard for the Quaternary of Eastern Asia 591: 87–92. doi:10.1016/j.quaint.2020.09.054. ISSN 1040-6182. https://www.sciencedirect.com/science/article/pii/S1040618220306194.
- ↑ Ghezzo, Elena; Boscaini, Alberto; Madurell-Malapeira, Joan; Rook, Lorenzo (2014-12-16). "Lynx remains from the Pleistocene of Valdemino cave (Savona, Northwestern Italy), and the oldest occurrence of Lynx spelaeus (Carnivora, Felidae)". Rendiconti Lincei 26 (2): 87–95. doi:10.1007/s12210-014-0363-4.
- ↑ Münzel, Susanne C.; Rivals, Florent; Pacher, Martina; Döppes, Doris; Rabeder, Gernot; Conard, Nicholas J.; Bocherens, Hervé (2014-08-07). "Behavioural ecology of Late Pleistocene bears (Ursus spelaeus, Ursus ingressus): Insight from stable isotopes (C, N, O) and tooth microwear". Quaternary International. Fossil remains in karst and their role in reconstructing Quaternary paleoclimate and paleoenvironments 339–340: 148–163. doi:10.1016/j.quaint.2013.10.020. Bibcode: 2014QuInt.339..148M.
- ↑ "Search for images at Natural History Museum Picture Library". http://piclib.nhm.ac.uk/results.asp.
- ↑ Harington, Charles Richard; Nature, Canadian Museum of (2003-01-01). Annotated Bibliography of Quaternary Vertebrates of Northern North America: With Radiocarbon Dates. University of Toronto Press. ISBN 9780802048172. https://books.google.com/books?id=BQtyg5m1zQkC.
- ↑ Turner, Alan (1997-01-01). The Big Cats and Their Fossil Relatives: An Illustrated Guide to Their Evolution and Natural History. Columbia University Press. ISBN 9780231102285. https://books.google.com/books?id=66mRJSxIAfoC.
- ↑ Tilson, Ronald; Nyhus, Philip J. (2009-11-30). Tigers of the World: The Science, Politics and Conservation of Panthera tigris. Academic Press. ISBN 9780080947518. https://books.google.com/books?id=XFIbjBEQolMC&q=panthera+tigris+beringia&pg=PA59.
- ↑ Martin, Paul S.; Klein, Richard G. (1989-01-01). Quaternary Extinctions: A Prehistoric Revolution. University of Arizona Press. ISBN 9780816511006. https://books.google.com/books?id=qIDC7ybvHQEC&q=cheetah+europe+pleistocene&pg=PA57.
- ↑ Kurtén, Björn (1968-01-01). Pleistocene Mammals of Europe. Transaction Publishers. ISBN 9781412845144. https://books.google.com/books?id=OsPBXSNL8ZkC&q=Asiatic+Cheetah+troy&pg=PA89.
- ↑ "English website FREE Nature – Wild waterbuffalo in holocene Europe". http://www.freenature.eu/free-en/p000429/news/news-archive/2012/wild-waterbuffalo-in-holocene-europe.
- ↑ "Pinguinus impennis (great auk)". http://animaldiversity.org/accounts/Pinguinus_impennis/.
- ↑ 122.00 122.01 122.02 122.03 122.04 122.05 122.06 122.07 122.08 122.09 122.10 122.11 122.12 122.13 Human origin sites and the World Heritage Convention in AmericasnVolume 1. 1. Sanz, Nuria. Paris. 31 December 2015. ISBN 9789231001406. OCLC 1002234186.
- ↑ Albarella, Umberto; Rizzetto, Mauro; Russ, Hannah; Viner-Daniels, Sarah (2017). The Oxford Handbook of Zooarchaeology. Oxford University Press. ISBN 9780199686476. https://books.google.com/books?id=wBcxDgAAQBAJ&q=Plyolimbus+baryosteus&pg=PA550.
- ↑ Palma-Ramírez, Arturo; Goyenechea, Irene; Castillo-Cerón, Jesús M. (2014-12-01). "Panbiogeography of the Santa María Amajac area, Hidalgo, Mexico". Revista Mexicana de Biodiversidad 85 (4): 1228–1234. doi:10.7550/rmb.44392.
- ↑ Arroyo-et-al-2008
- ↑ Pearson 2005
- ↑ Anthony D. Barnosky; Paul L. Koch; Robert S. Feranec; Scott L. Wing; Alan B. Shabel (2004). "Assessing the Causes of Late Pleistocene Extinctions on the Continents". Science 306 (5693): 70–75. doi:10.1126/science.1101476. PMID 15459379. Bibcode: 2004Sci...306...70B.
- ↑ Waters, Michael R.; Forman, Steven L.; Jennings, Thomas A.; Nordt, Lee C.; Driese, Steven G.; Feinberg, Joshua M.; Keene, Joshua L.; Halligan, Jessi et al. (2011-03-25). "The Buttermilk Creek Complex and the Origins of Clovis at the Debra L. Friedkin Site, Texas". Science 331 (6024): 1599–1603. doi:10.1126/science.1201855. ISSN 0036-8075. PMID 21436451. Bibcode: 2011Sci...331.1599W. https://semanticscholar.org/paper/7e0c5b0ba093748258cdc515ad47e72ee99b3f1d.
- ↑ 129.0 129.1 Romero, Simon (2014-03-27). "Discoveries Challenge Beliefs on Humans' Arrival in the Americas". The New York Times. ISSN 0362-4331. https://www.nytimes.com/2014/03/28/world/americas/discoveries-challenge-beliefs-on-humans-arrival-in-the-americas.html.
- ↑ "Humans in California 130,000 Years Ago? Get the Facts". 2017-04-26. http://news.nationalgeographic.com/2017/04/mastodons-americas-peopling-migrations-archaeology-science/.
- ↑ Fox-Dobbs, Kena; Leonard, Jennifer A.; Koch, Paul L. (2008). "Pleistocene megafauna from eastern Beringia: Paleoecological and paleoenvironmental interpretations of stable carbon and nitrogen isotope and radiocarbon records". Palaeogeography, Palaeoclimatology, Palaeoecology 261 (1–2): 30–46. doi:10.1016/j.palaeo.2007.12.011. Bibcode: 2008PPP...261...30F. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.710.5357&rep=rep1&type=pdf.
- ↑ Teresa Alberdi, Arroyo-Cabrales, Marín-Leyva, Polaco, María, Joaquín, Alejandro H., and Oscar J. (April 28, 2014). "Study of Cedral Horses and their place in the Mexican Quaternary". Revista Mexicana de Ciencias Geológicas. http://www.scielo.org.mx/pdf/rmcg/v31n2/v31n2a6.pdf.
- ↑ "Fossilworks: Equus complicatus". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=46261.
- ↑ "Fossilworks: Equus giganteus". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=46273.
- ↑ Guthrie, R. Dale (November 2013). Frozen Fauna of the Mammoth Steppe: The Story of Blue Babe. University of Chicago Press (published November 1, 2013). pp. 338. ISBN 9780226159713. https://books.google.com/books?id=KZnxAQAAQBAJ&q=equus+hemionus+beringia&pg=PA256.
- ↑ "Fossilworks: Equus (Asinus) kiang". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=162072.
- ↑ Teresa Alberdi, Arroyo-Cabrales, Marín-Leyva, Alberdi Polaco, María, Joaquín, Alejandro H., and Oscar J. (April 28, 2014). "Study of Cedral Horses and their place in the Mexican Quaternary". Revista Mexicana de Ciencias Geológicas. http://www.scielo.org.mx/pdf/rmcg/v31n2/v31n2a6.pdf.
- ↑ "Fossilworks: Equus pacificus". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=46295.
- ↑ Lundelius, Ernest L; Bryant, Vaughn M; Mandel, Rolfe; Thies, Kenneth J; Thoms, Alston (2013). "The First Occurrence of a Toxodont (Mammalia, Notoungulata) in the United States". Journal of Vertebrate Paleontology 33 (1): 229–232. doi:10.1080/02724634.2012.711405. https://www.researchgate.net/publication/260140036. Retrieved 2016-01-23.
- ↑ A New Occurrence of Toxodonts in the Pleistocene of México. 28. 2001. pp. 29–30. https://www.researchgate.net/publication/230820008. Retrieved 2016-01-23.
- ↑ "Fossilworks: Lynx lynx". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=160980.
- ↑ "Fossilworks: Enhydra macrodonta". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=46079.
- ↑ Anderson, E. (1973). "Ferrets from the pleistocene of central Alaska". J. Mammal. 54 (3): 778–779. doi:10.2307/1378982.
- ↑ 144.0 144.1 Schubert, Blaine W.; Chatters, James C.; Arroyo-Cabrales, Joaquin; Samuels, Joshua X.; Soibelzon, Leopoldo H.; Prevosti, Francisco J.; Widga, Christopher; Nava, Alberto et al. (2019-05-31). "Yucatán carnivorans shed light on the Great American Biotic Interchange". Biology Letters 15 (5): 20190148. doi:10.1098/rsbl.2019.0148. PMID 31039726.
- ↑ Youngman, Phillip M. (1986-03-01). "The extinct short-faced skunk Brachyprotoma obtusata (Mammalia, Carnivora): first records for Canada and Beringia". Canadian Journal of Earth Sciences 23 (3): 419–424. doi:10.1139/e86-043. Bibcode: 1986CaJES..23..419Y.
- ↑ Sanchez, Guadalupe; Holliday, Vance T.; Gaines, Edmund P.; Arroyo-Cabrales, Joaquín; Martínez-Tagüeña, Natalia; Kowler, Andrew; Lange, Todd; Hodgins, Gregory W. L. et al. (2014-07-29). "Human (Clovis)–gomphothere (Cuvieronius sp.) association ∼13,390 calibrated yBP in Sonora, Mexico". Proceedings of the National Academy of Sciences 111 (30): 10972–10977. doi:10.1073/pnas.1404546111. PMID 25024193. Bibcode: 2014PNAS..11110972S.
- ↑ Alberdi, María Teresa; Juárez-Woo, Javier; Polaco, Oscar J.; Arroyo-Cabrales, Joaquín (2009-02-01). "Description of the most complete skeleton of Stegomastodon (Mammalia, Gomphotheriidae) recorded for the Mexican Late Pleistocene". Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 251 (2): 239–255. doi:10.1127/0077-7749/2009/0251-0239.
- ↑ Phillips, Steven John; Comus, Patricia Wentworth; Dimmitt, Mark Alan; Brewer, Linda M. (2015-11-17). A Natural History of the Sonoran Desert. Univ of California Press. ISBN 9780520287471. https://books.google.com/books?id=cbQwDwAAQBAJ&q=giant+anteater+late+pleistocene&pg=PA68.
- ↑ "Fossilworks: Erethizon kleini". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=46322.
- ↑ "Fossilworks: Sylvilagus webbi". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=51920.
- ↑ Ceballos, Gerardo; Arroyo-Cabrales, Joaquín; Ponce, Eduardo (2010). "Effects of Pleistocene environmental changes on the distribution and community structure of the mammalian fauna of Mexico". Quaternary Research 73 (3): 464–473. doi:10.1016/j.yqres.2010.02.006. Bibcode: 2010QuRes..73..464C. https://www.researchgate.net/publication/220048614.
- ↑ Lucas, Spencer G.; Morgan, Gary S.; Spielmann, Justin A.; Prothero, Donald R. (2008). Neogene Mammals: Bulletin 44. New Mexico Museum of Natural History and Science. https://books.google.com/books?id=vW_aBwAAQBAJ&q=giant+anteater+late+pleistocene+honduras&pg=PA119.
- ↑ McDonald, H. Gregory; Chatters, James C.; Gaudin, Timothy J. (2017-05-04). "A new genus of megalonychid ground sloth (Mammalia, Xenarthra) from the late Pleistocene of Quintana Roo, Mexico". Journal of Vertebrate Paleontology 37 (3): e1307206. doi:10.1080/02724634.2017.1307206. ISSN 0272-4634.
- ↑ "Ice Age Predators Found Alongside Oldest Human in Americas". 2017-08-25. http://news.nationalgeographic.com/2017/08/ice-age-fossils-underwater-cave-bears-humans-science/.
- ↑ Stinnesbeck, Sarah R.; Frey, Eberhard; Olguín, Jerónimo Avíles; Stinnesbeck, Wolfgang; Zell, Patrick; Mallison, Heinrich; González, Arturo González; Núñez, Eugenio Aceves et al. (2017-06-01). "Xibalbaonyx oviceps, a new megalonychid ground sloth (Folivora, Xenarthra) from the Late Pleistocene of the Yucatán Peninsula, Mexico, and its paleobiogeographic significance". PalZ 91 (2): 245–271. doi:10.1007/s12542-017-0349-5. ISSN 0031-0220.
- ↑ "Ancient Giant Sloth Fossil Found in Underwater Cave". 2017-08-18. http://news.nationalgeographic.com/2017/08/ancient-giant-sloth-new-species-mexico-cave-spd/.
- ↑ Debus, Allen (June 2002). Dinosaur Memories. iUniverse. ISBN 9780595229888. https://books.google.com/books?id=oVl_ILVlbgIC&q=Doedicurus+habitat&pg=PA196.
- ↑ McDonough, Colleen M.; Loughry, W. J. (2013-03-18). The Nine-Banded Armadillo: A Natural History. University of Oklahoma Press. ISBN 9780806189215. https://books.google.com/books?id=h4UFvagbbogC.
- ↑ ondrej.zicha(at)gmail.com, Ondrej Zicha. "BioLib: Biological library". https://www.biolib.cz/en/taxon/id476718/.
- ↑ "Fossilworks: Phoenicopterus copei". http://fossilworks.org/?a=taxonInfo&taxon_no=335545.
- ↑ Feduccia, Alan (1999). The Origin and Evolution of Birds. Yale University Press. ISBN 978-0300078619. https://books.google.com/books?id=8QRKV7eSqmIC&q=Neophrontops&pg=PA300.
- ↑ Gillespie, Rosemary G.; Clague, D. A. (2009). Encyclopedia of Islands. University of California Press. ISBN 9780520256491. https://books.google.com/books?id=g9ZogGs_fz8C&q=Gigantohierax+suarezi&pg=PA321.
- ↑ Turvey, Sam (2009-05-28). Holocene Extinctions. OUP Oxford. ISBN 9780199535095. https://books.google.com/books?id=4QIUDAAAQBAJ&q=Gigantohierax+suarezi&pg=PA74.
- ↑ MacPhee, RDE (1999). Extinctions in Near Time: Causes, Contexts, and Consequences. Kluwer Academic Publishers. ISBN 978-0-306-46092-0.
- ↑ 165.0 165.1 Bell, C.J. (2004). "The Blancan, Irvingtonian, and Rancholabrean mammal ages". in Woodburne, M.O.. Late Cretaceous and Cenozoic Mammals of North America: Biostratigraphy and Geochronology. New York: Columbia Univ. Press. pp. 232–314. ISBN 978-0-231-13040-0.
- ↑ 166.0 166.1 Scott, E., Cox, S.M. (2008). "Late Pleistocene distribution of Bison (Mammalia; Artiodactyla) in the Mojave Desert of Southern California and Nevada". in Wang, X.. Geology and Vertebrate Paleontology of Western and Southern North America. Los Angeles: Natural History Museum of Los Angeles County. pp. 359–382.
- ↑ 167.0 167.1 Sanders, A.E., R.E. Weems, and L.B. Albright III (2009). "Formalization of the mid-Pleistocene "Ten Mile Hill beds" in South Carolina with evidence for placement of the Irvingtonian–Rancholabrean boundary". in Albright III, L.B.. Papers on Geology, Vertebrate Paleontology, and Biostratigraphy in Honor of Michael O. Woodburne. Flagstaff: Museum of Northern Arizona. pp. 369–375.
- ↑ 168.0 168.1 Shapiro, B. (2004). "Rise and Fall of the Beringian Steppe Bison". Science 306 (5701): 1561–1565. doi:10.1126/science.1101074. PMID 15567864. Bibcode: 2004Sci...306.1561S. http://summit.sfu.ca/item/15088.
- ↑ Wilson, M.C., L.V. Hills, and B. Shapiro (2008). "Late Pleistocene northward-dispersing Bos antiquus from the Bighill Creek Formation, Gallelli Gravel Pit, Alberta, Canada, and the fate of Bison occidentalis". Canadian Journal of Earth Sciences 45 (7): 827–859. doi:10.1139/E08-027. Bibcode: 2008CaJES..45..827W. https://semanticscholar.org/paper/b466a6d5391345bf6966a57bd326c8f433c7a59f.
- ↑ Kricher, John (2015-02-18). A Neotropical Companion: An Introduction to the Animals, Plants, and Ecosystems of the New World Tropics. Illustrated by Andrea S. LeJeune. Princeton University Press. ISBN 9781400866915. https://books.google.com/books?id=b2sZBgAAQBAJ&q=Doedicurus+central+america&pg=PA439.
- ↑ Coates, Anthony George (1999). Central America: A Natural and Cultural History. Yale University Press. ISBN 978-0300080650. https://books.google.com/books?id=jG_TV-akte8C&q=giant+anteater+late+pleistocene&pg=PA109.
- ↑ https://www.floridamuseum.ufl.edu/files/7513/9447/0046/bulletin-Mcdonaldlowres.pdf
- ↑ 173.0 173.1 Kurtén, Björn; Werdelin, Lars (1990). "Relationships between North and South American Smilodon". Journal of Vertebrate Paleontology 10 (2): 158–169. doi:10.1080/02724634.1990.10011804.
- ↑ 174.0 174.1 174.2 MacFadden, Bruce J. (2013-03-20). "Dispersal of Pleistocene Equus (Family Equidae) into South America and Calibration of GABI 3 Based on Evidence from Tarija, Bolivia". PLOS ONE 8 (3): e59277. doi:10.1371/journal.pone.0059277. ISSN 1932-6203. PMID 23527150. Bibcode: 2013PLoSO...859277M.
- ↑ 175.0 175.1 Jackson, Jeremy B. C.; Budd, Ann F.; Coates, Anthony G. (1996-12-15). Evolution and Environment in Tropical America. University of Chicago Press. ISBN 9780226389448. https://books.google.com/books?id=eJgcnw5p6qEC&q=Doedicurus+central+america&pg=PA344.
- ↑ Vizcaíno, Sergio F.; Bargo, M. Susana (2014-12-01). "Loss of Ancient Diversity of Xenarthrans and the Value of Protecting Extant Armadillos, Sloths and Anteaters". Edentata 15 (1): 27–38. doi:10.5537/020.015.0111. ISSN 1413-4411. http://naturalis.fcnym.unlp.edu.ar/repositorio/_documentos/sipcyt/bfa005306.pdf.
- ↑ 177.0 177.1 177.2 177.3 Oliveira, Édison V.; Porpino, Kleberson O.; Barreto, Alcina F. (2010). "On the presence of Glyptotherium in the Late Pleistocene of Northeastern Brazil, and the status of "Glyptodon" and "Chlamydotherium". Paleobiogeographic implications". Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen 258 (3): 353–363. doi:10.1127/0077-7749/2010/0116. https://www.researchgate.net/publication/233519649.
- ↑ Ortiz-Jaureguizar, E.; Cladera, G.A. (2006). "Paleoenvironmental evolution of southern South America during the Cenozoic". Journal of Arid Environments 66 (3): 498–532. doi:10.1016/j.jaridenv.2006.01.007. Bibcode: 2006JArEn..66..498O.
- ↑ Almeida, Rocha-dos-Santos, Bruno Cesar de; Scherer, Carolina Saldanha; Avilla, Leonardo dos Santos (2017). "fossil Camelidae (Mammalia: Cetartiodactyla) from the Gruta do Urso cave, northern Brazil" (in en). Quaternary International 436: 181–191. doi:10.1016/j.quaint.2017.01.025. ISSN 1040-6182. Bibcode: 2017QuInt.436..181R. http://agris.fao.org/agris-search/search.do?recordID=US201700082560.
- ↑ Cione, Alberto L.; Tonni, Eduardo P.; Soibelzon, Leopoldo (2009). "Did Humans Cause the Late Pleistocene-Early Holocene Mammalian Extinctions in South America in a Context of Shrinking Open Areas?". American Megafaunal Extinctions at the End of the Pleistocene. Vertebrate Paleobiology and Paleoanthropology. Springer, Dordrecht. pp. 125–144. doi:10.1007/978-1-4020-8793-6_7. ISBN 978-1-4020-8792-9.
- ↑ Elias, Scott; Mock, Cary (2013-03-25). Encyclopedia of Quaternary Science. Newnes. ISBN 9780444536426. https://books.google.com/books?id=TSTnzG6yetsC&q=Propaopus&pg=PT7768.
- ↑ Martin, Gabriela (1997) (in pt). Pré-história do Nordeste do Brasil. Editora Universitária UFPE. ISBN 9788573150834. https://books.google.com/books?id=exmtOsvSKj4C&q=Propaopus&pg=PA117.
- ↑ Figueroa, Natalia (2016). "Late Quaternary Megafaunal Extinctions in South America: Chronology, environmental changes and human impacts at regional scales". University of California, Berkeley. http://digitalassets.lib.berkeley.edu/etd/ucb/text/VillavicencioFigueroa_berkeley_0028E_16754.pdf.
- ↑ 184.0 184.1 184.2 Turvey, Samuel T. (2009-05-28). Holocene Extinctions. OUP Oxford. ISBN 9780191579981. https://books.google.com/books?id=mbU-F42JU1AC&q=Propaopus&pg=PA349.
- ↑ Veblen, Thomas T.; Young, Kenneth R.; Orme, Antony R. (2015-12-01). The Physical Geography of South America. Oxford University Press. ISBN 9780198031840. https://books.google.com/books?id=0Q-MY4-nlwwC&q=Doedicurus+central+america&pg=PA122.
- ↑ Hubbe, A.; Hubbe, M.; Neves, W. (2007-09-01). "Early Holocene survival of megafauna in South America". Journal of Biogeography 34 (9): 1642–1646. doi:10.1111/j.1365-2699.2007.01744.x. ISSN 1365-2699.
- ↑ MacPhee, Ross D. E.; SUES, HANS-DIETER (2013-11-09). Extinctions in Near Time: Causes, Contexts, and Consequences. Springer Science & Business Media. ISBN 9781475752021. https://books.google.com/books?id=nkLjBwAAQBAJ&q=Propaopus&pg=PA383.
- ↑ Borrero, Luis Alberto (2016-01-02). "Ambiguity and Debates on the Early Peopling of South America". PaleoAmerica 2 (1): 11–21. doi:10.1080/20555563.2015.1136498. ISSN 2055-5563.
- ↑ "Fossilworks: Agalmaceros". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=247645.
- ↑ "Fossilworks: Agalmaceros blicki". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=247647.
- ↑ "Fossilworks: Odocoileus salinae". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=247644.
- ↑ Pereira, Jamil Corrêa; Lopes, Renato Pereira; Kerber, Leonardo (2012). "New remains of Late Pleistocene mammals from the Chuí Creek, Southern Brazil". Revista Brasileira de Paleontologia 15 (2): 228–239. doi:10.4072/rbp.2012.2.10.
- ↑ Ransom, Jason I.; Kaczensky, Petra (2016-05-15). Wild Equids: Ecology, Management, and Conservation. JHU Press. ISBN 9781421419107. https://books.google.com/books?id=2vL-CwAAQBAJ&q=hippidion+california&pg=PA109.
- ↑ "Late Pleistocene vertebrates from Touro Passo Creek (Touro Passo Formation), southern Brazil: A review". Revista Mexicana de Ciencias Geológicas 31 (2): 248–259. https://www.researchgate.net/publication/265013705. Retrieved 2017-09-02.
- ↑ "Fossilworks: Macraucheniopsis". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=43498.
- ↑ Gaudioso, Pablo Javier; Gasparini, Germán M.; Herbst, Rafael; Barquez, Rubén Mario (2017-03-16). "First record of the Neolicaphrium recens Frenguelli, 1921 (Mammalia, Litopterna) in the Pleistocene of Santiago del Estero Province, Argentina". Papéis Avulsos de Zoologia 57 (3): 23–29. doi:10.11606/0031-1049.2017.57.03. ISSN 1807-0205.
- ↑ Prevosti, F. J.; Tonni, E. P.; Bidegain, J. C. (2009-12-01). "Stratigraphic range of the large canids (Carnivora, Canidae) in South America, and its relevance to quaternary biostratigraphy". Quaternary International. The Ensenadan Stage/Age in southern South America 210 (1): 76–81. doi:10.1016/j.quaint.2009.06.034. ISSN 1040-6182. Bibcode: 2009QuInt.210...76P.
- ↑ "Fossilworks: Valgipes". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=137597.
- ↑ Haynes, Gary (2008-12-23). American Megafaunal Extinctions at the End of the Pleistocene. Springer Science & Business Media. ISBN 9781402087936. https://books.google.com/books?id=iq6qZXUkWo0C&q=Arctotherium+bonariense&pg=PA126.
- ↑ Prothero, Donald R. (2016-11-15). The Princeton Field Guide to Prehistoric Mammals. Princeton University Press. ISBN 9781400884452. https://books.google.com/books?id=eiftDAAAQBAJ&q=Doedicurus+habitat&pg=PA54.
- ↑ Eisenberg, John F.; Redford, Kent H. (2000-05-15). Mammals of the Neotropics, Volume 3: Ecuador, Bolivia, Brazil. University of Chicago Press. ISBN 9780226195421. https://books.google.com/books?id=p2MDAzCeQQoC&q=Doedicurus+habitat&pg=PA17.
- ↑ Fariña, Richard A.; Vizcaíno, Sergio F.; Iuliis, Gerry De (2013-05-22). Megafauna: Giant Beasts of Pleistocene South America. Indiana University Press. ISBN 978-0253007193. https://books.google.com/books?id=kUAKgNfiAvoC&q=Doedicurus+habitat&pg=PA167.
- ↑ Martin, Paul Schultz (2005-01-01). Twilight of the Mammoths: Ice Age Extinctions and the Rewilding of America. University of California Press. ISBN 9780520231412. https://archive.org/details/twilightofmammot00paul.
- ↑ "Fossilworks: Neuryurus". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=43582.
- ↑ "Fossilworks: Parapanochthus". http://fossilworks.org/bridge.pl?a=taxonInfo&taxon_no=188888.
- ↑ Martin, Robert A.; Martin, Robert Allen; Barnosky, Anthony D. (2005-10-06). Morphological Change in Quaternary Mammals of North America. Cambridge University Press. ISBN 9780521020817. https://books.google.com/books?id=heAoqlZldWoC&q=chlamytherium+occidentale&pg=PA135.
- ↑ Turvey, Sam (2009-05-28). Holocene Extinctions. OUP Oxford. ISBN 9780199535095. https://books.google.com/books?id=4QIUDAAAQBAJ&q=chlamytherium+occidentale&pg=PA341.
- ↑ 208.0 208.1 Góis, Flávio; Ruiz, Laureano Raúl González; Scillato-Yané, Gustavo Juan; Soibelzon, Esteban (2015-06-17). "A Peculiar New Pampatheriidae (Mammalia: Xenarthra: Cingulata) from the Pleistocene of Argentina and Comments on Pampatheriidae Diversity". PLOS ONE 10 (6): e0128296. doi:10.1371/journal.pone.0128296. PMID 26083486. Bibcode: 2015PLoSO..1028296G.
- ↑ Jones, Washington; Rinderknecht, Andrés; Alvarenga, Herculano; Montenegro, Felipe; Ubilla, Martín (2017-12-30). "The last terror birds (Aves, Phorusrhacidae): new evidence from the late Pleistocene of Uruguay". PalZ 92 (2): 365–372. doi:10.1007/s12542-017-0388-y. ISSN 0031-0220.
- ↑ Alvarenga, Herculano; Jones, Washington; Rinderknecht, Andrés (2010-05-01). "The youngest record of phorusrhacid birds (Aves, Phorusrhacidae) from the late Pleistocene of Uruguay". Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 256 (2): 229–234. doi:10.1127/0077-7749/2010/0052.
- ↑ Agnolin, Federico (2013). "La posición sistemática de Hermosiornis (Aves, Phororhacoidea) y sus implicancias filogenéticas". Revista del Museo Argentino de Ciencias Naturales. Nueva Series 15 (1): 39–60. doi:10.22179/revmacn.15.167. ISSN 1853-0400.
- ↑ Jones, Washington; Rinderknecht, Andrés; Migotto, Rafael; Blanco, R. Ernesto (2013). "Body Mass Estimations and Paleobiological Inferences on a New Species of Large Caracara (Aves, Falconidae) from the Late Pleistocene of Uruguay". Journal of Paleontology 87 (1): 151–158. doi:10.1666/12-026R.1. https://www.researchgate.net/publication/260104736.
- ↑ Suárez, William; Olson, Storrs L. (2014-09-01). "A new fossil species of small crested caracara (Aves: Falconidae: Caracara) from the Pacific lowlands of western South America". Proceedings of the Biological Society of Washington 127 (2): 299–310. doi:10.2988/0006-324X-127.2.299. ISSN 0006-324X.
- ↑ "Fossilworks: Milvago brodkorbi". http://fossilworks.org/?a=taxonInfo&taxon_no=289782.
- ↑ 215.0 215.1 215.2 Harari, Yuval N. (2015). Sapiens A Brief History of Humankind. United States of America: HarperCollins. pp. 63–68. ISBN 978-0-06-231611-0.
- ↑ "Humans, not climate change, wiped out Australian megafauna" (in en). 2017-01-20. https://www.colorado.edu/today/2017/01/20/humans-not-climate-change-wiped-out-australian-megafauna.
- ↑ Wilson, Lito Vilisoni (2020-05-18). "Mysteries of megafauna extinction unlocked" (in en). https://about.unimelb.edu.au/newsroom/news/2020/may/mysteries-of-megafauna-extinction-unlocked.
- ↑ Hocknull, Scott A.; Lewis, Richard; Arnold, Lee J.; Pietsch, Tim; Joannes-Boyau, Renaud; Price, Gilbert J.; Moss, Patrick; Wood, Rachel et al. (2020-05-18). "Extinction of eastern Sahul megafauna coincides with sustained environmental deterioration" (in en). Nature Communications 11 (1): 2250. doi:10.1038/s41467-020-15785-w. ISSN 2041-1723. PMID 32418985. Bibcode: 2020NatCo..11.2250H.
- ↑ Saltré, Frédérik; Chadoeuf, Joël; Peters, Katharina J.; McDowell, Matthew C.; Friedrich, Tobias; Timmermann, Axel; Ulm, Sean; Bradshaw, Corey J. A. (2019-11-22). "Climate-human interaction associated with southeast Australian megafauna extinction patterns" (in en). Nature Communications 10 (1): 5311. doi:10.1038/s41467-019-13277-0. ISSN 2041-1723. PMID 31757942. Bibcode: 2019NatCo..10.5311S.
- ↑ Martin, Paul S.; Klein, Richard G. (1989-01-01). Quaternary Extinctions: A Prehistoric Revolution. University of Arizona Press. ISBN 9780816511006. https://books.google.com/books?id=qIDC7ybvHQEC&q=Phascolomys+medius&pg=PA622.
- ↑ 221.0 221.1 221.2 Lourandos, Harry (1997-02-28). Continent of Hunter-Gatherers: New Perspectives in Australian Prehistory. Cambridge University Press. ISBN 9780521359467. https://books.google.com/books?id=tTy-I8no1MwC&q=Phascolomys+medius&pg=PA99.
- ↑ 222.0 222.1 222.2 MacPhee, R. D. E. (1999-06-30). Extinctions in Near Time. Springer Science & Business Media. ISBN 9780306460920. https://books.google.com/books?id=UZLuF1YXYTcC&q=Euryzygoma&pg=PA251.
- ↑ Long, John A.; Archer, Michael (2002-01-01). Prehistoric Mammals of Australia and New Guinea: One Hundred Million Years of Evolution. UNSW Press. ISBN 9780868404356. https://books.google.com/books?id=LwMkO0M1mPQC&q=Warendja+wakefieldi&pg=PA116.
- ↑ 224.0 224.1 Tyndale-Biscoe, Hugh (2005-04-22). Life of Marsupials. Csiro Publishing. ISBN 9780643099210. https://books.google.com/books?id=YALY-B6s7IEC&q=Warendja+wakefieldi&pg=PA269.
- ↑ Webb, Steve (2013-02-27). Corridors to Extinction and the Australian Megafauna. Newnes. ISBN 9780124078406. https://books.google.com/books?id=C0blLvmdp04C&q=Phascolomys+medius&pg=PA279.
- ↑ 226.0 226.1 "Megafauna". http://austhrutime.com/marsupial_megafauna.htm.
- ↑ 227.0 227.1 "Anaspides.net". http://www.anaspides.net/earth_life_sciences/megafauna_extinction.html.
- ↑ 228.0 228.1 Webb, Steve (2013-02-27). Corridors to Extinction and the Australian Megafauna. Newnes. ISBN 9780124078406. https://books.google.com/books?id=C0blLvmdp04C&q=Warendja+wakefieldi&pg=PA279.
- ↑ 229.0 229.1 Long, John A.; Archer, Michael (2002-01-01). Prehistoric Mammals of Australia and New Guinea: One Hundred Million Years of Evolution. UNSW Press. ISBN 9780868404356. https://books.google.com/books?id=LwMkO0M1mPQC&q=Troposodon+minor&pg=PA163.
- ↑ Society, Australian Mammal (1981-05-13). Australian Mammal Society. Australian Mammal Society. https://books.google.com/books?id=lIYw_r7_waAC&q=Phascolomys+medius&pg=PA71.
- ↑ 231.0 231.1 231.2 Bayly, I. a. E. (1993-01-01). "The fauna of athalassic saline waters in Australia and the Altiplano of South America: Comparisons and historical perspectives". in Hurlbert, Stuart H.. Saline Lakes V. Developments in Hydrobiology. Springer Netherlands. pp. 225–231. doi:10.1007/978-94-011-2076-0_18. ISBN 9789401049214.
- ↑ Doughty, C. E.; Wolf, A.; Field, C. B. (2010). "Biophysical feedbacks between the Pleistocene megafauna extinction and climate: The first human‐induced global warming?". Geophys. Res. Lett. 37 (15): L15703. doi:10.1029/2010GL043985. Bibcode: 2010GeoRL..3715703D.
- ↑ Grayson, Donald K.; Meltzer, David J. (December 2012). "Clovis Hunting and Large Mammal Extinction: A Critical Review of the Evidence". Journal of World Prehistory 16 (4): 313–359. doi:10.1023/A:1022912030020.
- ↑ Kolbert, Elizabeth (2014). The Sixth Extinction: An Unnatural History. Bloomsbury Publishing. ISBN 9781408851210.
- ↑ Perry, George L. W.; Wheeler, Andrew B.; Wood, Jamie R.; Wilmshurst, Janet M. (2014-12-01). "A high-precision chronology for the rapid extinction of New Zealand moa (Aves, Dinornithiformes)". Quaternary Science Reviews 105: 126–135. doi:10.1016/j.quascirev.2014.09.025. Bibcode: 2014QSRv..105..126P.
- ↑ Crowley, Brooke E. (2010-09-01). "A refined chronology of prehistoric Madagascar and the demise of the megafauna". Quaternary Science Reviews. Special Theme: Case Studies of Neodymium Isotopes in Paleoceanography 29 (19–20): 2591–2603. doi:10.1016/j.quascirev.2010.06.030. Bibcode: 2010QSRv...29.2591C.
- ↑ Li, Sophia (2012-09-20). "Has Plant Life Reached Its Limits?". http://green.blogs.nytimes.com/2012/09/20/has-plant-life-reached-its-limits/?_r=0.
- ↑ Martin P. S. (1963). The last 10,000 years: A fossil pollen record of the American Southwest. Tucson, AZ: Univ. Ariz. Press. ISBN 978-0-8165-1759-6.
- ↑ Martin P. S. (1967). "Prehistoric overkill". in Martin, P.S.. Pleistocene extinctions: The search for a cause. New Haven: Yale Univ. Press. ISBN 978-0-300-00755-8.
- ↑ Martin P. S. (1989). "Prehistoric overkill: A global model". in Martin, P.S.. Quaternary extinctions: A prehistoric revolution. Tucson, AZ: Univ. Arizona Press. pp. 354–404. ISBN 978-0-8165-1100-6.
- ↑ Martin, P. S. (2005). Twilight of the Mammoths: Ice Age Extinctions and the Rewilding of America. University of California Press. ISBN 978-0-520-23141-2. https://archive.org/details/twilightofmammot00paul.
- ↑ Burney, D. A.; Flannery, T. F. (July 2005). "Fifty millennia of catastrophic extinctions after human contact". Trends in Ecology & Evolution 20 (7): 395–401. doi:10.1016/j.tree.2005.04.022. PMID 16701402. http://www.anthropology.hawaii.edu/Fieldschools/Kauai/Publications/Publication%204.pdf.
- ↑ Diamond J (2008). "Palaeontology: The last giant kangaroo". Nature 454 (7206): 835–6. doi:10.1038/454835a. PMID 18704074. Bibcode: 2008Natur.454..835D.
- ↑ "Late-surviving megafauna in Tasmania, Australia, implicate human involvement in their extinction". Proc. Natl. Acad. Sci. U.S.A. 105 (34): 12150–3. 26 August 2008. doi:10.1073/pnas.0801360105. PMID 18719103. Bibcode: 2008PNAS..10512150T.
- ↑ 245.0 245.1 The future eaters: an ecological history of the Australasian lands and people. New York: Grove/Atlantic, Inc.. 2002-10-16. ISBN 978-0-8021-3943-6. OCLC 32745413. http://google.com/books?id=eIW5aktgo0IC&printsec=frontcover.
- ↑ Diamond, J. (1984). "Historic extinctions: a Rosetta stone for understanding prehistoric extinctions". in Martin, P.S.. Quaternary extinctions: A prehistoric revolution. Tucson, AZ: Univ. Arizona Press. pp. 824–62. ISBN 978-0-8165-1100-6.
- ↑ Diamond, J. (1997). Guns, germs, and steel; the fates of human societies. New York: Norton. ISBN 978-0-393-31755-8.
- ↑ Mossiman, J. E.; Martin, P. S. (1975). "Simulating Overkill by Paleoindians". American Scientist 63 (3): 304–13. Bibcode: 1975AmSci..63..304M.
- ↑ Whittington, S. L.; Dyke, B. (1984). "Simulating overkill: experiment with the Mossiman and Martin model". in Martin, P.S.. Quaternary extinctions: A prehistoric revolution. Tucson, AZ: Univ. Arizona Press. pp. 451–66. ISBN 978-0-8165-1100-6.
- ↑ 250.0 250.1 Alroy, J. (2001). "A multispecies overkill simulation of the end-Pleistocene megafaunal mass extinction". Science 292 (5523): 1893–6. doi:10.1126/science.1059342. PMID 11397940. Bibcode: 2001Sci...292.1893A. http://www.nceas.ucsb.edu/~alroy/pdfs/2001-Science-292-1893.pdf.
- ↑ 251.0 251.1 251.2 "Humans responsible for demise of gigantic ancient mammals". University of Exeter. 13 August 2015. https://www.exeter.ac.uk/news/featurednews/title_465673_en.html.
- ↑ 252.0 252.1 Lewis J. Bartlett, David R. Williams, Graham W. Prescott, Andrew Balmford, Rhys E. Green, Anders Eriksson, Paul J. Valdes, Joy S. Singarayer, Andrea Manica (2016). "Robustness despite uncertainty: regional climate data reveal the dominant role of humans in explaining global extinctions of Late Quaternary megafauna". Ecography 39 (2): 152–161. doi:10.1111/ecog.01566. https://research-information.bristol.ac.uk/en/publications/robustness-despite-uncertainty(94b9d1da-e339-47d9-803e-fa5a64cff28c).html.
- ↑ Martin, Paul S. (1966). "Africa and Pleistocene overkill". Nature 212 (5060): 339–342. doi:10.1038/212339a0. Bibcode: 1966Natur.212..339M. http://max2.ese.u-psud.fr/epc/conservation/PDFs/HIPE/Martin1966.pdf.
- ↑ May, R. M. (2001). Stability and complexity in model ecosystems. 6. Princeton: Princeton Univ. Press. 1–235. ISBN 978-0-691-08861-7.
- ↑ Grayson, D.K.; Meltzer, D.J. (2003). "A requiem for North American overkill". Journal of Archaeological Science 30 (5): 585–593. doi:10.1016/s0305-4403(02)00205-4.
- ↑ Fiedel, S., Haynes, G., 2004. A premature burial: comments on Grayson and Meltzer’s Requiem for overkill Journal of Archaeological Science 31, 121–131.
- ↑ Scott, J. (2009-02-26). "Camel-butchering in Boulder, 13,000 years ago". University of Colorado at Boulder. http://artsandsciences.colorado.edu/magazine/2009/02/camel-butchering-in-boulder-13000-years-ago/.
- ↑ Surovell, Todd A; Brigid S Grund (2012). "The associational critique of Quaternary overkill". American Antiquity 77 (4): 673–688. doi:10.7183/0002-7316.77.4.672. https://semanticscholar.org/paper/825e7bae0435ecedd10e93826db025be1ba8822a.
- ↑ Wilsom, M.C., L.V. Hills, and B. Shapiro (2008). "Late Pleistocene northward-dispersing Bison antiquus from the Bighill Creek Formation, Gallelli Gravel Pit, Alberta, Canada, and the fate of Bison occidentalis". Canadian Journal of Earth Sciences 45 (7): 827–859. doi:10.1139/E08-027. Bibcode: 2008CaJES..45..827W. https://semanticscholar.org/paper/b466a6d5391345bf6966a57bd326c8f433c7a59f.
- ↑ Nadasdy, Paul (2006). "Transcending the Debate over the Ecologically Noble Indian: Indigenous Peoples and Environmentalism". Ethnohistory 52 (2): 291–331. doi:10.1215/00141801-52-2-291. https://semanticscholar.org/paper/36e690791be0a600e1041dc508fbf13a21ea456f.
- ↑ "Buffalo Jump." Wikipedia.
- ↑ 262.0 262.1 Scott, E. (2010). "Extinctions, scenarios, and assumptions: Changes in latest Pleistocene large herbivore abundance and distribution in western North America". Quat. Int. 217 (1–2): 225–239. doi:10.1016/j.quaint.2009.11.003. Bibcode: 2010QuInt.217..225S.
- ↑ Willis, Paul; Bryce, Clay; Searle, Mike (17 August 2006). "Thylacoleo — The Beast of the Nullarbor". Catalyst. Australian Broadcasting Commissionhttp://www.abc.net.au/catalyst/stories/s1717424.htm
|transcript-url=missing title (help). - ↑ "An arid-adapted middle Pleistocene vertebrate fauna from south-central Australia". Nature 445 (7126): 422–5. January 2007. doi:10.1038/nature05471. PMID 17251978. Bibcode: 2007Natur.445..422P.
- ↑ "New ages for the last Australian megafauna: continent-wide extinction about 46,000 years ago". Science 292 (5523): 1888–92. June 2001. doi:10.1126/science.1060264. PMID 11397939. Bibcode: 2001Sci...292.1888R. https://semanticscholar.org/paper/7284ed470065c2c53db88a3bddd7de6f945a8595.
- ↑ Louys, Julien; Curnoe, D.; Tong, H. (2007). "Characteristics of Pleistocene megafauna extinctions in Southeast Asia". Palaeogeography, Palaeoclimatology, Palaeoecology 243 (1–2): 152–173. doi:10.1016/j.palaeo.2006.07.011.
- ↑ Fisher, Daniel C. (2009). "Paleobiology and Extinction of Proboscideans in the Great Lakes Region of North America". in Haynes, Gary. American Megafaunal Extinctions at the End of the Pleistocene. Vertebrate Paleobiology and Paleoanthropology. Springer. pp. 55–75. doi:10.1007/978-1-4020-8793-6_4. ISBN 978-1-4020-8792-9. https://semanticscholar.org/paper/375bc6559355a9bdae547a6ab8556d4dbecd2b4f.
- ↑ 268.0 268.1 Rabanus-Wallace, M. Timothy; Wooller, Matthew J.; Zazula, Grant D.; Shute, Elen; Jahren, A. Hope; Kosintsev, Pavel; Burns, James A.; Breen, James et al. (2017). "Megafaunal isotopes reveal role of increased moisture on rangeland during late Pleistocene extinctions". Nature Ecology & Evolution 1 (5): 0125. doi:10.1038/s41559-017-0125. PMID 28812683.
- ↑ Andersen, S. T (1973). "The differential pollen productivity of trees and its significance for the interpretation of a pollen diagram from a forested region". in Birks, H.J.B.. Oxford: Blackwell Scientific. ISBN 0-632-09120-7.
- ↑ Ashworth, C.A. (1980). "Environmental implications of a beetle assemblage from the Gervais formation (Early Wisconsinian?), Minnesota". Quat. Res. 13 (2): 200–12. doi:10.1016/0033-5894(80)90029-0. Bibcode: 1980QuRes..13..200A.
- ↑ 271.0 271.1 Birks, H.H. (1973). "Modern macrofossil assemblages in lake sediments in Minnesota". in Birks, H.J.B.. Oxford: Blackwell Scientific. ISBN 0-632-09120-7.
- ↑ 272.0 272.1 Birks, H.J.B., Birks, H.H. (1980). Quaternary paleoecology. Baltimore: Univ. Park Press. ISBN 978-1-930665-56-9.
- ↑ Bradley, R. S. (1985). Quaternary Paleoclimatology: Methods of Paleoclimatic Reconstruction. Winchester, MA: Allen & Unwin. ISBN 978-0-04-551068-9. https://archive.org/details/quaternarypaleoc0000unse.
- ↑ 274.0 274.1 Davis, M. B. (1976). "Pleistocene biogeography of temperate deciduous forests". Geoscience and man: Ecology of the Pleistocene. 13. Baton Rouge: School of Geoscience, Louisiana State Univ..
- ↑ Vartanyan, S.L., Arslanov, K.A., Tertychnaya, T.V. & Chernov, S.B. (1995). "Radiocarbon dating evidence for mammoths on Wrangel Island, Arctic Ocean, until 2000 BC". Radiocarbon 37: 1–6. doi:10.1017/S0033822200014703. https://journals.uair.arizona.edu/index.php/radiocarbon/article/viewFile/1640/1644.
- ↑ Guthrie, R. D. (1988). Frozen Fauna of the Mammoth Steppe: The Story of Blue Babe. University Of Chicago Press. ISBN 978-0-226-31122-7.
- ↑ Guthrie, R. D. (1989). "Mosaics, allochemics, and nutrients: an ecological theory of Late Pleistocene megafaunal extinctions". in Martin, P.S.. Quaternary extinctions: A prehistoric revolution. Tucson, AZ: Univ. Arizona Press. pp. 259–99. ISBN 978-0-8165-1100-6.
- ↑ Hoppe, P.P. (1978). "Rumen fermentation in African ruminants". Atlanta.
- ↑ Bryson, R.A., Baerreis, D.A., Wendland, W.M. (1970). "The character of late-glacial and post-glacial climatic changes". in Dort Jr., W.. Pleistocene and recent environments of the central Great Plains. Lawrence: Univ. Press Kan. Univ. Kan. Spec. Pub. 3. ISBN 978-0-7006-0063-2. https://archive.org/details/pleistocenerecen0000symp.
- ↑ Graham, R.W., Lundelius, E.L. (1989). "Coevolutionary disequilibrium and Pleistocene extinctions". in Martin, P.S.. Quaternary extinctions: A prehistoric revolution. Tucson, AZ: Univ. Arizona Press. pp. 354–404. ISBN 978-0-8165-1100-6.
- ↑ King, J.E., Saunders, J.J. (1989). "Environmental insularity and the extinction of the American mastodont". in Martin, P.S.. Quaternary extinctions: A prehistoric revolution. Tucson, AZ: Univ. Arizona Press. pp. 354–404. ISBN 978-0-8165-1100-6.
- ↑ Axelrod, D. I. (1967). "Quaternary extinctions of large mammals". University of California Publications in Geological Sciences 74: 1–42. ASIN B0006BX8LG.
- ↑ Slaughter, B. H. (1967). "Animal ranges as a clue to late-Pleistocene extinction". in Martin, P.S.. Pleistocene extinctions: The search for a cause. New Haven: Yale Univ. Press. ISBN 978-0-300-00755-8.
- ↑ Kilti, R. A. (1988). "Seasonality, gestation time, and large mammal extinctions". in Martin, P.S.. Quaternary extinctions: A prehistoric revolution. Tucson, AZ: Univ. Arizona Press. pp. 354–404. ISBN 978-0-8165-1100-6.
- ↑ Flereov, C.C. (1967). "On the origin of the mammalian fauna of Canada". in Hopkins, D.M.. The Bering Land Bridge. Palo Alto: Stanford Univ. Press. pp. 271–80. ISBN 978-0-8047-0272-0. https://archive.org/details/beringlandbridge0000hopk.
- ↑ Frenzel, B. (1968). "The Pleistocene vegetation of northern Eurasia". Science 161 (3842): 637–49. doi:10.1126/science.161.3842.637. PMID 17801456. Bibcode: 1968Sci...161..637F.
- ↑ 287.0 287.1 McDonald, J. (1989). "The reordered North American selection regime and late Quaternary megafaunal extinctions". in Martin, P.S.. Quaternary extinctions: A prehistoric revolution. Tucson, AZ: Univ. Arizona Press. pp. 354–404. ISBN 978-0-8165-1100-6.
- ↑ Birks, H.J.B., West, R.G. (1973). "A Symposium of the British Ecological Society". Oxford: Blackwell Scientific. ISBN 0-632-09120-7.
- ↑ McDonald, J. (1981). North American Bison: Their classification and evolution. Berkeley: Univ. Calif. Press. ISBN 978-0-520-04002-1.
- ↑ Burney, D. A. (1993). "Recent animal extinctions: recipes for disaster". American Scientist 81 (6): 530–41. Bibcode: 1993AmSci..81..530B.
- ↑ Vartanyan, S.L., Garutt, V. E. and Sher, A.V. (1993). "Holocene dwarf mammoths from Wangel Island in the Siberian Arctic". Nature 362 (6418): 337–40. doi:10.1038/362337a0. PMID 29633990. Bibcode: 1993Natur.362..337V.
- ↑ Pennycuick, C.J. (1979). "Energy costs of locomotion and the concept of "Foraging radius"". Serengetti: Dynamics of an Ecosystem. Chicago: Univ. Chicago Press. pp. 164–85. ISBN 978-0-226-76029-2.
- ↑ Wing, L.D., Buss, I.O. (1970). "Elephants and Forests". Wildl. Mong. (19).
- ↑ Owen-Smith, R.N. (1992). Megaherbivores: The influence of very large body size on ecology. Cambridge studies in ecology. Cambridge: Cambridge Univ. Press. ISBN 978-0-521-42637-4.
- ↑ Kershaw, G.P. (1984). "Tundra plant communities of the Mackenzie mountains, Northwest Territories; floristic characteristics of long term surface disturbances". in Olson, R.. Northern Ecology and Resource Management: Memorial Essays honoring Don Gill. Edmonton, Canada: Univ. Alberta Press. pp. 239–311. ISBN 978-0-88864-047-5. https://archive.org/details/northernecologyr0000unse/page/239.
- ↑ Webber, P.J., Miller, P.C., Chapin, F.S. III, MacCown, B.H. (1980). "The vegetation: pattern and succession". in Brown, J.. An Arctic ecosystem: the coastal tundra at Barrow, Alaska. US/IBP Synthesis. Stroudsburg, PA: Dowden Hutchinson & Ross. pp. 186–219. 12.
- ↑ Whitney-Smith, Elin (2008). "The Evolution of an Ecosystem: Pleistocene Extinctions". Unifying themes in complex systems IV proceedings of the Fourth International Conference on Complex Systems. Springer. ISBN 978-3-540-73849-7. https://www.researchgate.net/publication/237242493.
- ↑ 298.0 298.1 Whitney-Smith, E. (2006). Clovis and Extinctions – Overkill, Second Order Predation, Environmental Degradation in a Non-equilibrium Ecosystem "Clovis Age Continent". University of New Mexico Press.
- ↑ Whitney-Smith, Elin (2012). "Creating the tiniest bison: A system dynamics model of ecological evolution". Ruminants: Anatomy, Behavior, and Diseases. Nova Biomedical. ISBN 9781620810644. https://www.researchgate.net/publication/286116200.
- ↑ 300.0 300.1 MacFee, Ross D. E.; Marx, Preston A. (1997). "Humans, hyperdisease and first-contact extinctions". in Goodman, S.; Patterson, B. D.. Natural Change and Human Impact in Madagascar. Washington DC: Smithsonian Press. pp. 169–217. ISBN 978-1-56098-683-6.
- ↑ MacFee, Ross D. E.; Marx, Preston A. (1998). "Lightning Strikes Twice: Blitzkrieg, Hyperdisease, and Global Explanations of the Late Quaternary Catastrophic Extinctions". American Museum of Natural History. http://www.amnh.biz/science/biodiversity/extinction/Day1/bytes/MacPheePres.html.
- ↑ MacFee, Ross D. E.; Marx, Preston A. (1997). "The 40,000-year Plague: Humans, Hyperdisease, and First-contact Extinctions". Washington DC: Smithsonian Institution Press. pp. 169–217.
- ↑ Fiedel, S. (2005). "Man's best friend: mammoth's worst enemy?". World Archaeology 37: 11–35. doi:10.1080/0043824042000329540. https://zenodo.org/record/895416.
- ↑ Lyons, K.; Smith, F. A.; Wagner, P. J.; White, E. P.; Brown, J. H. (2004). "Was a 'hyperdisease' responsible for the late Pleistocene megafaunal extinction?". Ecology Letters 7 (9): 859–68. doi:10.1111/j.1461-0248.2004.00643.x. http://biology.unm.edu/fasmith/Web_Page_PDFs/Lyons_et_al_2004_WN.pdf.
- ↑ Whitney-Smith, E. (2004). "Late Pleistocene extinctions through second-order predation". in Barton, C.M.. Settlement of the American Continents: A Multidisciplinary Approach to Human Biogeography. Tucson, AZ: University of Arizona Press. ISBN 978-0-8165-2323-8.
- ↑ Whitney-Smith, E. (2009). The Second-Order Predation Hypothesis of Pleistocene Extinctions: A System Dynamics Model. Saarbruken, Germany: VDM Verlag. ISBN 978-3-639-11579-6.
- ↑ Scott, E. (2010). "Extinctions, scenarios, and assumptions: Changes in latest Pleistocene large herbivore abundance and distribution in western North America". Quat. Int.
- ↑ Study links mammoth extinction, comets, USA Today, Updated 1/2/2009. Retrieved 4 April 2009
- ↑ The Extinction Debate, by Evan Hadingham, NOVA, Retrieved 4 April 2009.
- ↑ Last Extinction, NOVA, TV Program Description, Original PBS Broadcast Date: March 31, 2009, Retrieved 4 April 2009.
- ↑ Petaev, M. I.; Huang, S.; Jacobsen, S. B.; Zindler, A. (2013-08-06). "Large Pt anomaly in the Greenland ice core points to a cataclysm at the onset of Younger Dryas". Proceedings of the National Academy of Sciences (PNAS) 110 (32): 12917–12920. doi:10.1073/pnas.1303924110. PMID 23878232. Bibcode: 2013PNAS..11012917P.
- ↑ Moore, Christopher R.; West, Allen; LeCompte, Malcolm A.; Brooks, Mark J.; Daniel, I. Randolph; Goodyear, Albert C.; Ferguson, Terry A.; Ivester, Andrew H. et al. (2017-03-09). "Widespread platinum anomaly documented at the Younger Dryas onset in North American sedimentary sequences". Scientific Reports 7 (1): 44031. doi:10.1038/srep44031. ISSN 2045-2322. PMID 28276513. Bibcode: 2017NatSR...744031M.
- ↑ Wolbach, Wendy S.; Ballard, Joanne P.; Mayewski, Paul A.; Parnell, Andrew C.; Cahill, Niamh; Adedeji, Victor; Bunch, Ted E.; Domínguez-Vázquez, Gabriela et al. (March 2018). "Extraordinary Biomass-Burning Episode and Impact Winter Triggered by the Younger Dryas Cosmic Impact ∼12,800 Years Ago. 2. Lake, Marine, and Terrestrial Sediments". The Journal of Geology 126 (2): 185–205. doi:10.1086/695704. ISSN 0022-1376. Bibcode: 2018JG....126..185W. https://semanticscholar.org/paper/95973f135b3919130883692b8e50466c9d5d0417.
- ↑ Anderson, David G.; Smallwood, Ashley M.; Miller, D. Shane (2015). "Pleistocene Human Settlement in the Southeastern United States: Current Evidence and Future Directions". PaleoAmerica 1: 7–51. doi:10.1179/2055556314Z.00000000012.
- ↑ "Paleoindian demography and the extraterrestrial impact hypothesis". Proc. Natl. Acad. Sci. U.S.A. 105 (33): 11651–4. 19 August 2008. doi:10.1073/pnas.0803762105. PMID 18697936. Bibcode: 2008PNAS..10511651B.
- ↑ Haynes, Gary (2009). American megafaunal extinctions at the end of the Pleistocene. Springer. p. 125. ISBN 978-1-4020-8792-9. https://books.google.com/books?id=iq6qZXUkWo0C&pg=PA125.
- ↑ Anderson, David G.; Goodyear, Albert C.; Kennett, James; West, Allen (2011). "Multiple lines of evidence for possible Human population decline/settlement reorganization during the early Younger Dryas". Quaternary International 242 (2): 570–583. doi:10.1016/j.quaint.2011.04.020. ISSN 1040-6182. Bibcode: 2011QuInt.242..570A.
- ↑ 318.0 318.1 318.2 Haynes, Gary (2009). "Introduction to the Volume". in Haynes, Gary. American Megafaunal Extinctions at the End of the Pleistocene. Vertebrate Paleobiology and Paleoanthropology. Springer. pp. 1–20. doi:10.1007/978-1-4020-8793-6_1. ISBN 978-1-4020-8792-9. https://semanticscholar.org/paper/dbe0d2a8dcb6e3c2174a718684a64cf7ffb97491.
- ↑ 319.0 319.1 319.2 Fiedel, Stuart (2009). "Sudden Deaths: The Chronology of Terminal Pleistocene Megafaunal Extinction". in Haynes, Gary. American Megafaunal Extinctions at the End of the Pleistocene. Vertebrate Paleobiology and Paleoanthropology. Springer. pp. 21–37. doi:10.1007/978-1-4020-8793-6_2. ISBN 978-1-4020-8792-9.
- ↑ 320.0 320.1 Vergano, Dan (2009-01-02). "Study links mammoth extinction, comets". Gannett Company. https://www.usatoday.com/tech/science/2009-01-01-mammothimpact_N.htm.
- ↑ Roach, John (2010-06-22). "Fungi, Feces Show Comet Didn't Kill Ice Age Mammals?". National Geographic Society. http://news.nationalgeographic.com/news/2010/06/100622-science-environment-wildfires-cooling-ice-age-extinctions/.
- ↑ Daulton, T. L.; Pinter, N.; Scott, A. C. (2010-08-30). "No evidence of nanodiamonds in Younger–Dryas sediments to support an impact event". Proc. Natl. Acad. Sci. U.S.A. 107 (37): 16043–7. doi:10.1073/pnas.1003904107. PMID 20805511. Bibcode: 2010PNAS..10716043D.
- ↑ Kerr, Richard A. (2010-10-30). "Mammoth-Killer Impact Rejected". AAAS. http://news.sciencemag.org/sciencenow/2010/08/mammoth-killer-impact-rejected.html.
- ↑ "Nanodiamonds do not provide unique evidence for a Younger Dryas impact". Proc. Natl. Acad. Sci. U.S.A. 108 (1): 40–4. January 2011. doi:10.1073/pnas.1007695108. PMID 21173270. Bibcode: 2011PNAS..108...40T.
- ↑ Bement, Leland C.; Madden, Andrew S.; Carter, Brian J.; Simms, Alexander R.; Swindle, Andrew L.; Alexander, Hanna M.; Fine, Scott; Benamara, Mourad (2014-02-04). "Quantifying the distribution of nanodiamonds in pre-Younger Dryas to recent age deposits along Bull Creek, Oklahoma Panhandle, USA". Proceedings of the National Academy of Sciences 111 (5): 1726–1731. doi:10.1073/pnas.1309734111. ISSN 0027-8424. PMID 24449875. Bibcode: 2014PNAS..111.1726B.
- ↑ Israde-Alcántara, Isabel; Bischoff, James L.; DeCarli, Paul S.; Domínguez-Vázquez, Gabriela; Bunch, Ted E.; Firestone, Richard B.; Kennett, James P.; West, Allen (2012-08-21). "Reply to Blaauw et al., Boslough, Daulton, Gill et al., and Hardiman et al.: Younger Dryas impact proxies in Lake Cuitzeo, Mexico". Proceedings of the National Academy of Sciences 109 (34): E2245–E2247. doi:10.1073/pnas.1209463109. ISSN 0027-8424. Bibcode: 2012PNAS..109E2245I.
- ↑ LeCompte, Malcolm A.; Batchelor, Dale; Demitroff, Mark N.; Vogel, Edward K.; Mooney, Charles; Rock, Barrett N.; Seidel, Alfred W. (2013-04-30). "Reply to Boslough: Prior studies validating research are ignored". Proceedings of the National Academy of Sciences 110 (18): E1652. doi:10.1073/pnas.1300425110. ISSN 0027-8424. PMID 23762911. Bibcode: 2013PNAS..110E1652L.
- ↑ Kennett, James P.; Kennett, Douglas J.; Culleton, Brendan J.; Tortosa, J. Emili Aura; Bunch, Ted E.; Erlandson, Jon M.; Johnson, John R.; Pardo, Jesús F. Jordá et al. (2015-12-08). "Reply to Holliday and Boslough et al.: Synchroneity of widespread Bayesian-modeled ages supports Younger Dryas impact hypothesis". Proceedings of the National Academy of Sciences 112 (49): E6723–E6724. doi:10.1073/pnas.1520411112. ISSN 0027-8424. PMID 26604309. Bibcode: 2015PNAS..112E6723K.
- ↑ LeCompte, Malcolm A.; Goodyear, Albert C.; Demitroff, Mark N.; Batchelor, Dale; Vogel, Edward K.; Mooney, Charles; Rock, Barrett N.; Seidel, Alfred W. (2012-10-30). "Independent evaluation of conflicting microspherule results from different investigations of the Younger Dryas impact hypothesis". Proceedings of the National Academy of Sciences 109 (44): E2960–E2969. doi:10.1073/pnas.1208603109. ISSN 0027-8424. PMID 22988071.
- ↑ Napier, William M.; Bunch, Ted E.; Kennett, James P.; Wittke, James H.; Tankersley, Kenneth B.; Kletetschka, Gunther; Howard, George A.; West, Allen (2013-11-05). "Reply to Boslough et al.: Decades of comet research counter their claims". Proceedings of the National Academy of Sciences 110 (45): E4171. doi:10.1073/pnas.1315467110. ISSN 0027-8424. PMID 24350338. Bibcode: 2013PNAS..110E4171N.
- ↑ Broecker, Wallace (September 13, 2018). "Broecker Briefs". https://www.ldeo.columbia.edu/~broecker/Home.html.
External links
Hyperdisease hypothesis
- "American Museum of Natural History". http://www.amnh.biz/science/biodiversity/extinction/Day1/bytes/MacPheePres.html.
- J.H. Brown (University of New Mexico). "Was a hyperdisease responsible?". http://biology.unm.edu/JHBrown/Published/WasAHyperdiseaseResponsible.x.pdf.
Second-order predation
- Elin Whitney-Smith. "Quaternary.Net". http://quaternary.net.
Other links
- "Ice Age Bay Area". http://www.kqed.org/quest/television/ice-age-bay-area2.
- "The Extinct Late Pleistocene Mammals of North America". https://www.pbs.org/wgbh/nova/stoneage/mega-list.html.
- Peter Tyson. "End of the Big Beasts". https://www.pbs.org/wgbh/nova/beta/evolution/end-big-beasts.html.
- S. Kathleen Lyons; Felisa A. Smith; James H. Brown (2004). "Of mice, mastodons and men: human-mediated extinctions on four continents". Evolutionary Ecology Research 6: 339–358. http://biology.unm.edu/fasmith/Web_Page_PDFs/Lyons_et_al_2004_EER.pdf.
- "Return to the Ice Age: The La Brea Exploration Guide". http://www.tarpits.org/education/guide/index.html.