Chemistry:Aquayamycin
From HandWiki
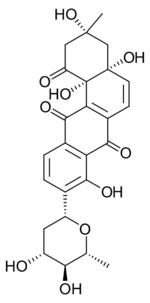
| |
| Names | |
|---|---|
| Preferred IUPAC name
(3R,4aR,12bS)-9-[(2R,4R,5S,6R)-4,5-Dihydroxy-6-methyloxan-2-yl]-3,4a,8,12b-tetrahydroxy-3-methyl-3,4,4a,12b-tetrahydrotetraphene-1,7,12(2H)-trione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | aquayamycin |
PubChem CID
|
|
| |
| |
| Properties | |
| C25H26O10 | |
| Molar mass | 486.47 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Aquayamycin is an anthraquinone derivative.[2] It is an inhibitor of the enzyme tyrosine hydroxylase.
Saquayamycins (saquayamycins A, B, C and D) are antibiotics of the aquayamycin group found in Streptomyces nodosus cultures broth.[3]
References
- ↑ Aquayamycin - Compound Summary, PubChem.
- ↑ Sezaki, M.; Kondo, S.; Maeda, K.; Umezawa, H.; Ono, M. (1970). "The structure of aquayamycin". Tetrahedron 26 (22): 5171–5190. doi:10.1016/S0040-4020(01)98726-5. PMID 5499897.
- ↑ Uchida, T.; Imoto, M.; Watanabe, Y.; Miura, K.; Dobashi, T.; Matsuda, N.; Sawa, T.; Naganawa, H. et al. (1985). "Saquayamycins, new aquayamycin-group antibiotics". The Journal of Antibiotics 38 (9): 1171–1181. doi:10.7164/antibiotics.38.1171. PMID 3840796.
 |

