Chemistry:Difluorophosphoric acid
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Difluorophosphinic acid[1]
| |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 1768 |
| |
| Properties | |
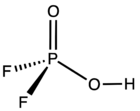
| HPO 2F 2 | |
| Molar mass | 101.977 g·mol−1 |
| Appearance | Colorless liquid[1] |
| Density | 1.583 g/cm3[1][2] |
| Melting point | −96.5 °C (−141.7 °F; 176.7 K)[2] |
| Boiling point | 115.9 °C (240.6 °F; 389.0 K)[2] |
| Structure | |
| Tetrahedral at phosphorus atom | |
| Hazards | |
| Main hazards | Corrosive to living tissue |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H314 | |
| P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Difluorophosphoric acid is an inorganic compound with the formula HPO
2F
2. It is a mobile colorless strongly fuming liquid.[1] The acid has limited applications, in part because it is thermally and hydrolytically unstable.[3] Difluorophosphoric acid is corrosive to glass, fabric, metals and living tissue.[1]
A method to make pure difluorphosphoric acid involves heating phosphoryl fluoride with fluorophosphoric acid and separating the product by distillation:[4]
- POF
3 + H
2PO
3F → 2 HPO
2F
2
It is prepared by hydrolysis of phosphoryl fluoride:
- POF
3 + H
2O → HPO
2F
2 + HF
Further hydrolysis gives fluorophosphoric acid:
- HPO
2F
2 + H
2O → H
2PO
3F + HF
Complete hydrolysis gives phosphoric acid:
- H
2PO
3F + H
2O → H
3PO
4 + HF
The salts of difluorophosphoric acid are known as difluorophosphates.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 https://pubchem.ncbi.nlm.nih.gov/compound/Difluorophosphoric-acid
- ↑ 2.0 2.1 2.2 Reed, William (September 1965). Studies of Difluorophosphoric Acid and its Alkali Metal Salts (Thesis). Retrieved 23 April 2023.
- ↑ Charles B. Lindahl; Tariq Mahmood (2000). "Fluorine Compounds, Inorganic, Phosphorus". Kirk‐Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.1608151912091404.a01. ISBN 0-471-23896-1.
- ↑ Lange, Willy; Livingston, Ralph (March 1950). "Studies of Fluorophosphoric Acids and their Derivatives. XIV. Preparation of Anhydrous Difluorophosphoric Acid". Journal of the American Chemical Society 72 (3): 1280–1281. doi:10.1021/ja01159a057.
 |

