Chemistry:Tetraethylammonium diiron oxyhexachloride
From HandWiki
| |
| Names | |
|---|---|
| Other names
bis(tetraethylammonium) (μ-oxo)bis[trichloroferrate(III)]
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| C16H40Cl6Fe2N2O | |
| Molar mass | 600.90 g·mol−1 |
| Appearance | yellow-brown solid |
| Density | 1.354 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
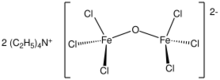
Tetraethylammonium diiron oxyhexachloride is the chemical compound with the formula (N(C2H5)4)2Fe2OCl6. It is the tetraethylammonium salt of [Fe2OCl6]2-. Many related salts of [Fe2OCl6]2- are known. The anion consists of a pair of tetrahedral Fe(III) centers that share a oxo bridging ligand.[2] The salt can be prepared by treatment of tetraethylammonium tetrachloroferrate with sodium trimethylsiloxide.[1]
References
- ↑ 1.0 1.1 Do, Y.; Simhon, E. D.; Holm, R. H. (1983). "Improved Syntheses of Tetrachlorodi-μ-sulfidodiferrate Dianion ([Fe2S2Cl4]2-) and Hexachloro-μ-oxodiferrate2- ([Fe2OCl6]2-) and Oxo/Sulfido Ligand Substitution by Use of Silylsulfide Reagents". Inorg. Chem. 22: 3809-12. doi:10.1021/ic00167a027.μ
- ↑ Haselhorst, Gabriele; Wieghardt, Karl; Keller, Stefan; Schrader, Bernhard (1993). "The (μ-Oxo)bis[trichloroferrate(III)] Dianion Revisited". Inorganic Chemistry 32 (5): 520–525. doi:10.1021/ic00057a006.
 |

