Chemistry:Mesalazine: Difference between revisions
(fixing) |
(No difference)
|
Latest revision as of 05:01, 6 February 2024
 | |
| Clinical data | |
|---|---|
| Trade names | Asacol, Lialda, Pentasa, others[1] |
| Other names | mesalamine, 5-aminosalicylic acid, 5-ASA, Mesalamine (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a688021 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, rectal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | orally: 20–30% absorbed rectally: 10–35% |
| Metabolism | Rapidly & extensively metabolized intestinal mucosal wall and the liver |
| Elimination half-life | 5 hours after initial dose. At steady state 7 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
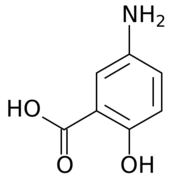
| Formula | C7H7NO3 |
| Molar mass | 153.137 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 283 °C (541 °F) |
| |
| |
| (verify) | |
Mesalazine, also known as mesalamine or 5-aminosalicylic acid (5-ASA), is a medication used to treat inflammatory bowel disease, including ulcerative colitis and Crohn's disease.[1] It is generally used for mildly to moderately severe disease.[1] It is taken by mouth or rectally.[1] The formulations which are taken by mouth appear to be similarly-effective.[9]
Common side-effects include headache, nausea, abdominal pain, and fever.[1] Serious side-effects may include pericarditis, liver problems, and kidney problems.[1][9] Use in pregnancy and breastfeeding appears safe.[9] In people with a sulfa allergy certain formulations may result in problems.[1] Mesalazine is an aminosalicylate and anti-inflammatory.[1][9] It works by direct contact with the intestines.[1]
Mesalazine was approved for medical use in the United States in 1987.[1][5] It is on the World Health Organization's List of Essential Medicines.[10] It is available as a generic medication.[1][11][12][13] In 2021, it was the 239th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[14][15]
Medical uses
It is used to treat inflammatory bowel disease, including ulcerative colitis and Crohn's disease (effective only in colonic diseases).[1] It is generally used for mildly to moderately active disease.[1] It is taken by mouth or rectally.[1] The formulations which are taken by mouth appear to be similarly effective.[9]
In 2022 Germany introduced guidance to use mesalamine to treat acute uncomplicated diverticulitis.[16]
Side-effects
Most often reported side-effects are gastrointestinal (GI) (but may also include headache), including: nausea, diarrhea, and abdominal pain.[4]
Very rarely, use of mesalazine has been associated with an exacerbation of the symptoms of colitis, Stevens Johnson syndrome, and erythema multiforme.[4]
Pregnancy
There is no data on use in pregnant women, but the drug does cross the placenta and is excreted in breast milk. The drug should not be used in children under two years of age,[4] people with kidney disease,[4] or people who are allergic to aspirin.[4]
Chemistry
Mesalazine is the active moiety of sulfasalazine, which is metabolized to sulfapyridine and mesalazine.[17] It is also the active component of the prodrug balsalazide along with the inert carrier molecule 4-aminobenzoyl-beta-alanine.[18] It is in the category of disease-modifying antirheumatic drugs (DMARDs) family of medications.[19] It is unclear exactly how it works.[19] Mesalazine is claimed to be a PPAR-γ agonist.[20]
Mechanism of action
Exact mechanism of mesalazine is unknown, but is speculated that mesalazine decreases synthesis of prostaglandin and leukotriene, modulating the inflammatory response derived from the cyclooxygenase and lipooxygenase pathways.[21] It appears to act locally on colonic mucosa.[22]
Society and culture
Brand names
Mesalazine is sold under various names including Apriso, Asacol, Asacol HD, Canasa, Delzicol, Fivasa, Lialda, Pentasa, Rowasa, Octasa, and Sfrowasa.[23][24]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 "Mesalamine Monograph for Professionals". American Society of Health-System Pharmacists. https://www.drugs.com/monograph/mesalamine.html.
- ↑ "Mesalamine Use During Pregnancy". 18 September 2018. https://www.drugs.com/pregnancy/mesalamine.html.
- ↑ "Mesalazine Sun/ Mesalz (Sun Pharma ANZ Pty Ltd)". 13 January 2023. https://www.tga.gov.au/resources/prescription-medicines-registrations/mesalazine-sun-mesalz-sun-pharma-anz-pty-ltd.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 "Asacol 400mg MR Tablets - Summary of Product Characteristics (SmPC)". 14 April 2016. https://www.medicines.org.uk/emc/medicine/12583.
- ↑ 5.0 5.1 "Asacol HD- mesalamine tablet, delayed release". 15 April 2018. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2f68f68c-58d2-4575-b573-f2e62f95d7e3.
- ↑ "Pentasa- mesalamine capsule". 8 November 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e39d9a3d-5d3a-4bb6-aab1-fdbb2a598606.
- ↑ "Lialda- mesalamine tablet, delayed release". 8 November 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3098a080-be86-4265-9818-7fc4beab77b7.
- ↑ Human Medicines Evaluation Division (15 October 2020). "Active substance: mesalazine". List of nationally authorised medicinal products. European Medicines Agency. https://www.ema.europa.eu/documents/psusa/mesalazine-list-nationally-authorised-medicinal-products-psusa/00001990/202002_en.pdf.
- ↑ 9.0 9.1 9.2 9.3 9.4 British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 39–41. ISBN 9780857113382.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ "ANDA Approval Reports - 2017 First Generic Drug Approvals". 3 November 2018. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/ANDAGenericDrugApprovals/ucm597322.htm.
- ↑ "2022 First Generic Drug Approvals". 3 March 2023. https://www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/2022-first-generic-drug-approvals.
- ↑ "Competitive Generic Therapy Approvals". 29 June 2023. https://www.fda.gov/drugs/generic-drugs/competitive-generic-therapy-approvals.
- ↑ "The Top 300 of 2021". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Mesalamine - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Mesalamine.
- ↑ Peery, Anne F. (November 12, 2022). "New German guidelines for the management of diverticulitis". United European Gastroenterology Journal 10 (9): 913–914. doi:10.1002/ueg2.12331. PMID 36302089.
- ↑ Lippincott's Illustrated Reviews: Pharmacology (Fourth ed.). Lippincott Williams & Wilkins. 2009. ISBN 978-0-7817-7155-9.
- ↑ "Balsalazide: increasing the choice for patients with ulcerative colitis.". Drugs & Therapy Perspectives 19 (1–4): 1–4. 2003. doi:10.2165/00042310-200319100-00001.
- ↑ 19.0 19.1 "Sulfasalazine". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/sulfasalazine.html.
- ↑ "5-Aminosalicylic Acid Ameliorates Colitis and Checks Dysbiotic Escherichia coli Expansion by Activating PPAR-γ Signaling in the Intestinal Epithelium". mBio 12 (1): e03227–20. January 2021. doi:10.1128/mBio.03227-20. PMID 33468700.
- ↑ "Mesalamine (USAN)". StatPearls. Treasure Island (FL): StatPearls Publishing. 2022. http://www.ncbi.nlm.nih.gov/books/NBK551714/. Retrieved 1 September 2022.
- ↑ "Mesalazine in inflammatory bowel disease: a trendy topic once again?". Canadian Journal of Gastroenterology 24 (2): 127–133. February 2010. doi:10.1155/2010/586092. PMID 20151072.
- ↑ "Substance Name: Mesalamine [USAN:USP"]. https://chem.nlm.nih.gov/chemidplus/rn/89-57-6.
- ↑ "Mesalamine Uses, Side Effects & Warnings". 30 August 2019. https://www.drugs.com/mtm/mesalamine.html.
 |

