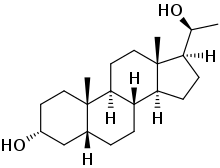
Chemistry:Pregnanediol
| |
| Names | |
|---|---|
| IUPAC name
(3R,5R,8R,9S,10S,13S,14S,17S)-17-[(1S)-1-Hydroxyethyl]-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol
| |
| Other names
5β-Pregnane-3α,20α-diol; Pregnandiol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H36O2 | |
| Molar mass | 320.517 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pregnanediol, or 5β-pregnane-3α,20α-diol, is an inactive metabolic product of progesterone. A test can be done to measure the amount of pregnanediol in urine, which offers an indirect way to measure progesterone levels in the body.[1]
From the urine of pregnant women from London clinics, Guy Frederic Marrian isolated a substance that contained two hydroxyl groups and could be converted into a diacetate with acetic anhydride. However, the formula had not been clearly clarified.[2] Almost at the same time, Adolf Butenandt at the Chemical University Laboratory in Göttingen investigated the constituents of pregnant urine and clarified the structure of the diol.[3] The name pregnandiol, coined by Butenandt, is derived from the Latin verb praegnans (pregnant) or the English pregnant and pregnancy. This gave rise to the name pregnane for the underlying parent hydrocarbon.
In 1936, Venning and Browne demonstrated the presence of pregnanediol, specifically the glucuronide of pregnanediol in pregnancy urine. Their study extracted pregnanediol from pregnancy urine and revealed that pregnanediol concentration in urine indicates the amount of progesterone excreted. Since progesterone levels indicate the functionality of a corpus luteum, and pregnanediol concentration represents 40-45% of the progesterone excreted, estimations of pregnanediol reveal the functionality of a corpus luteum. However, pregnanediol concentrations vary with menstrual cycle phases, so it is essential to consider the menstrual cycle phase when examining them.[4] Furthermore, current research has demonstrated that pregnanediol concentration in urine is also a measure of ovarian activity.[5]
Chemistry
See also
- Pregnanedione
- Progesterone (pregn-4-ene-3,20-dione)
References
- ↑ Smith, Melanie N. (May 1, 2007). "Pregnanediol". A.D.A.M., Inc.. https://www.nytimes.com/health/guides/test/pregnanediol/overview.html.
- ↑ Marrian, Guy Frederic (1929-01-01). "The chemistry of oestrin". Biochemical Journal 23 (5): 1090–1098. doi:10.1042/bj0231090. ISSN 0306-3283. PMID 16744278. PMC 1254231. http://dx.doi.org/10.1042/bj0231090.
- ↑ Butenandt, Adolf (1930-03-05). "Über das Pregnandiol, einen neuen Sterin-Abkömmling aus Schwangeren-Harn". Berichte der Deutschen Chemischen Gesellschaft (A and B Series) 63 (3): 659–663. doi:10.1002/cber.19300630319. ISSN 0365-9488. http://dx.doi.org/10.1002/cber.19300630319.
- ↑ Hain, A.; Robertson, Edwin (June 17, 1939). "Pregnanediol Excretion in the Menstrual Cycle". The British Medical Journal 1 (4093): 1226–1228. doi:10.1136/bmj.1.4093.1226. PMID 20782425. PMC 2209830. https://www-ncbi-nlm-nih-gov/pmc/articles/PMC2209830/.
- ↑ Blackwell, Leonard; Cooke, Delwyn; Brown, Simon (May 31, 2018). "The Use of Estrone-3-Glucuronide and Pregnanediol-3-Glucuronide Excretion Rates to Navigate the Continuum of Ovarian Activity". Frontiers in Public Health 6: 153. doi:10.3389/fpubh.2018.00153. PMID 29904626. PMC 5990994. https://www-ncbi-nlm-nih-gov./pmc/articles/PMC5990994/.
 |

