Chemistry:Sulfur trifluoride
From HandWiki
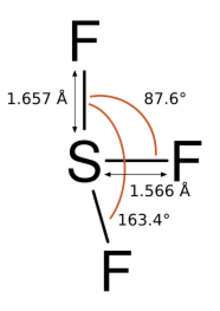
| |
| Names | |
|---|---|
| Other names
sulfur(III) fluoride
trifluorosulfur radical | |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| F3S | |
| Molar mass | 89.06 g·mol−1 |
| Related compounds | |
Related compounds
|
SF2, SF4, SF6, S2F10 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sulfur trifluoride is the inorganic chemical compound with the formula SF3. It is a radical.[1][2]
Structure and synthesis
Sulfur trifluoride is predicted to be pyramidal.[3][4]
SF3 is generated by irradiation of crystals SF+3BF−4 with gamma rays.[1]
SF−3
A derivative formally derived from SF−3 is the coordination complex Ir(Cl)(CO)(F)(SF3)(Et3P)2 obtained by oxidative addition of sulfur tetrafluoride to Ir(Cl)(CO)(PEt3)2 (Et = C2H5).[5][6]
References
- ↑ 1.0 1.1 Morton, J. R.; Preston, K. F.; Strach, S. J. (1978). "The EPR spectrum of SF3 Trapped in SF3BF4 Crystals". The Journal of Chemical Physics 69 (4): 1392. doi:10.1063/1.436766. Bibcode: 1978JChPh..69.1392M.
- ↑ Deng, Jianming; Wang, Chaoyang; Li, Qian-shu; Xie, Yaoming; King, R. Bruce; Schaefer, Henry F. (2011). "Trifluorosulfane Ligand as an Analogue of the Nitrosyl Ligand: Highly Exothermic Fluorine Transfer Reactions from Sulfur to Metal in the Chemistry of SF3 Metal Carbonyls of the First Row Transition Metals". Inorganic Chemistry 50 (7): 2824–2835. doi:10.1021/ic101994k. PMID 21366337.
- ↑ Irikura, Karl K. (1995). "Structure and thermochemistry of sulfur fluorides SFn (n = 1–5) and their ions SF+n (n = 1–5)". The Journal of Chemical Physics 102 (13): 5357–5367. doi:10.1063/1.469263. Bibcode: 1995JChPh.102.5357I. https://zenodo.org/record/1232101.
- ↑ Woon, David E.; Dunning, Thom H. (2009). "Theory of Hypervalency: Recoupled Pair Bonding in SFn (n = 1−6)". The Journal of Physical Chemistry A 113 (27): 7915–7926. doi:10.1021/jp901949b. PMID 19499905. Bibcode: 2009JPCA..113.7915W.
- ↑ Cockman, Russell W.; Ebsworth, E. A. V.; Holloway, John H. (1987). "Complexes of iridium(III) containing the novel sulfur trifluoride ligand". Journal of the American Chemical Society 109 (7): 2194–2195. doi:10.1021/ja00241a055.
- ↑ Gao, Xiaozhen; Li, Nan; King, R. Bruce (December 2014). "Formation of Difluorosulfane Complexes of the Third Row Transition Metals by Sulfur-to-Metal Fluorine Migration in Trifluorosulfane Metal Complexes: The Anomaly of Trifluorosulfane Iridium Tricarbonyl". Inorganic Chemistry 53 (23): 12635–12642. doi:10.1021/ic502375q. PMID 25397720.
 |

