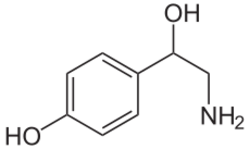
Biology:Octopamine (neurotransmitter)
 | |
| Clinical data | |
|---|---|
| Other names | OCT, Norsympathol, Norsynephrine, para-Octopamine, beta-Hydroxytyramine, para-hydroxy-phenyl-ethanolamine |
| Physiology data | |
| Source tissues | invertebrate nervous systems; trace amine in vertebrates |
| Target tissues | system-wide in invertebrates |
| Receptors | TAAR1 (mammals) OctαR, OctβR, TyrR (invertebrates) |
| Precursor | tyramine |
| Biosynthesis | tyramine β-hydroxylase; dopamine β-hydroxylase |
| Metabolism | N-acetyltransferases; phenylethanolamine N-methyltransferase |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| KEGG | |
Octopamine is an organic chemical closely related to norepinephrine, and synthesized biologically by a homologous pathway. Its name derives from the fact that it was first identified in the salivary glands of the octopus.
In many types of invertebrates octopamine is an important neurotransmitter and hormone. In protostomes—arthropods, molluscs, and several types of worms—it substitutes for norephinephrine and performs functions apparently similar to those of norepinephrine in mammals, functions that have been described as mobilizing the body and nervous system for action.
Functions
Cellular effects
Octopamine exerts its effects by binding to and activating receptors located on the surface of cells. These receptors have mainly been studied in insects, where they can be divided into three types: alpha-adrenergic-like (OctαR), which are structurally and functionally similar to noradrenergic alpha-1 receptors in mammals; beta-adrenergic-like (OctβR), which are structurally and functionally similar to noradrenergic beta receptors in mammals; and mixed octopamine/tyramine receptors (TyrR), which are structurally and functionally similar to noradrenergic alpha-2 receptors in mammals.[1] Receptors in the TyrR class, however, are generally more strongly activated by tyramine than by octopamine.[1]
In vertebrates no octopamine-specific receptors have been identified. Octopamine binds weakly to receptors for norepinephrine and epinephrine, but it is not clear whether this has any functional significance. It binds more strongly to trace amine-associated receptors (TAARs), especially TAAR1.[1]
Invertebrates
Octopamine was first discovered by Italian scientist Vittorio Erspamer in 1948[2] in the salivary glands of the octopus and has since been found to act as a neurotransmitter, neurohormone and neuromodulator in invertebrates. Although Erspamer discovered its natural occurrence and named it, octopamine had actually existed for many years as a pharmaceutical product.[3] It is widely used in energy-demanding behaviors by all insects, crustaceans (crabs, lobsters, crayfish), and spiders. Such behaviors include flying, egg-laying, and jumping.
Octopamine acts as the insect equivalent of norepinephrine and has been implicated in regulating aggression in invertebrates, with different effects on different species. Studies have shown that reducing the neurotransmitter octopamine and preventing coding of tyramine beta hydroxylase (an enzyme that converts tyramine to octopamine) decreases aggression in Drosophila without influencing other behaviors.[4]
The best-understood role for octopamine is in the locust jump. Here it modulates muscle activity, making the leg muscles contract more effectively. This is at least in part due to an increase in the rate of contraction and of relaxation.[citation needed] Octopamine, also has a role in locust flight. A study done in 2014 showed that when a locust was injected with octopamine the acoustic startle response of a locust during flight was changed, and the flight path of the locust was erratic.
In the honey bee and fruit fly, octopamine has a major role in learning and memory. In the firefly, octopamine release leads to light production in the lantern.[5][6]
Octopamine also plays a role in mollusks, though the role of octopamine has been examined only in the central nervous system of the model organism, the pond snail.[citation needed]
In lobsters, octopamine seems to direct and coordinate neurohormones to some extent in the central nervous system, and it was observed that injecting octopamine into a lobster and crayfish resulted in limb and abdomen extension.[7]
Heberlein et al.[8] have conducted studies of alcohol tolerance in fruit flies; they found that a mutation that caused octopamine deficiency also caused lower alcohol tolerance.[9][10][11][12]
The emerald cockroach wasp stings the host for its larvae (a cockroach) in the head ganglion (brain). The venom blocks octopamine receptors[13] and the cockroach fails to show normal escape responses, grooming itself excessively. It becomes docile and the wasp leads it to the wasp's den by pulling its antenna like a leash.[14]
Vertebrates
In vertebrates, octopamine replaces norepinephrine in sympathetic neurons with chronic use of monoamine oxidase inhibitors. It may be responsible for the common side effect of orthostatic hypotension with these agents, though there is also evidence that it is actually mediated by increased levels of N-acetylserotonin.
One study noted that octopamine might be an important amine that influences the therapeutic effects of inhibitors such as monoamine oxidase inhibitors, especially because a large increase in octopamine levels was observed when animals were treated with this inhibitor. Octopamine was positively identified in the urine samples of mammals such as humans, rats, and rabbits treated with monoamine oxidase inhibitors. Very small amounts of octopamine were also found in certain animal tissues. It was observed that within a rabbit's body, the heart and kidney held the highest concentrations of octopamine.[3]
Biochemical mechanisms
See also
References
- ↑ 1.0 1.1 1.2 Pflüger HJ, Stevensonb PA (2005). "Evolutionary aspects of octopaminergic systems with emphasis on arthropods". Arthropod Structure & Development 34: 379–396. doi:10.1016/j.asd.2005.04.004. https://www.researchgate.net/profile/Hans_Pflueger/publication/235927256_Evolutionary_aspects_of_octopamine_systems_with_emphasis_on_arthropods/links/0deec52f385fc005f4000000.pdf.
- ↑ Erspamer, V. (2009). "Active Substances in the Posterior Salivary Glands of Octopoda. II. Tyramine and Octopamine (Oxyoctopamine)". Acta Pharmacologica et Toxicologica 4 (3–4): 224–47. doi:10.1111/j.1600-0773.1948.tb03345.x.
- ↑ 3.0 3.1 Kakimoto, Y; Armstrong, M. D. (1962). "On the identification of octopamine in mammals". The Journal of Biological Chemistry 237: 422–7. PMID 14453200.
- ↑ Zhou, Chuan; Rao, Yong; Rao, Yi (2008). "A subset of octopaminergic neurons are important for Drosophila aggression". Nature Neuroscience 11 (9): 1059–67. doi:10.1038/nn.2164. PMID 19160504.
- ↑ Greenfield, M. D. (November 2001). "Missing link in firefly bioluminescence revealed: NO regulation of photocyte respiration". BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology 23 (11): 992–995. doi:10.1002/bies.1144. ISSN 0265-9247. PMID 11746215.
- ↑ Trimmer, B. A.; Aprille, J. R.; Dudzinski, D. M.; Lagace, C. J.; Lewis, S. M.; Michel, T.; Qazi, S.; Zayas, R. M. (2001-06-29). "Nitric oxide and the control of firefly flashing". Science 292 (5526): 2486–2488. doi:10.1126/science.1059833. ISSN 0036-8075. PMID 11431567.
- ↑ Livingstone, M. S.; Harris-Warrick, R. M.; Kravitz, E. A. (1980). "Serotonin and Octopamine Produce Opposite Postures in Lobsters". Science 208 (4439): 76–9. doi:10.1126/science.208.4439.76. PMID 17731572. Bibcode: 1980Sci...208...76L.
- ↑ Heberlein, U.; Wolf, F. W.; Rothenfluh, A; Guarnieri, D. J. (2004). "Molecular Genetic Analysis of Ethanol Intoxication in Drosophila melanogaster". Integrative and Comparative Biology 44 (4): 269–74. doi:10.1093/icb/44.4.269. PMID 21676709.
- ↑ Moore, Monica S; Dezazzo, Jim; Luk, Alvin Y; Tully, Tim; Singh, Carol M; Heberlein, Ulrike (1998). "Ethanol Intoxication in Drosophila: Genetic and Pharmacological Evidence for Regulation by the cAMP Signaling Pathway". Cell 93 (6): 997–1007. doi:10.1016/S0092-8674(00)81205-2. PMID 9635429.
- ↑ Tecott, Laurence H; Heberlein, Ulrike (1998). "Y Do We Drink?". Cell 95 (6): 733–5. doi:10.1016/S0092-8674(00)81695-5. PMID 9865690.
- ↑ Williams, Ruth (June 22, 2005). "Bar Flies: What our insect relatives can teach us about alcohol tolerance". Naked Scientist. http://www.thenakedscientists.com/HTML/articles/article/ruthwilliamscolumn1.htm/.
- ↑ Vince, Gaia (22 August 2005). "'Hangover gene' is key to alcohol tolerance". New Scientist. https://www.newscientist.com/article.ns?id=dn7830.
- ↑ Hopkin, Michael (2007). "How to make a zombie cockroach". Nature. doi:10.1038/news.2007.312.
- ↑ Gal, Ram; Rosenberg, Lior Ann; Libersat, Frederic (2005). "Parasitoid wasp uses a venom cocktail injected into the brain to manipulate the behavior and metabolism of its cockroach prey". Archives of Insect Biochemistry and Physiology 60 (4): 198–208. doi:10.1002/arch.20092. PMID 16304619.

