Chemistry:N-Acetylaspartic acid
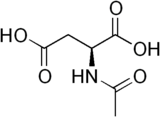
| |
| Names | |
|---|---|
| IUPAC name
2-Acetamidobutanedioic acid[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 1726198 S | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | N-acetylaspartate |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C6H9NO5 | |
| Molar mass | 175.140 g·mol−1 |
| Appearance | Colourless, transparent crystals |
| Melting point | 137 to 140 °C (279 to 284 °F; 410 to 413 K) |
| Boiling point | 141 to 144 °C (286 to 291 °F; 414 to 417 K) |
| log P | −2.209 |
| Acidity (pKa) | 3.142 |
| Basicity (pKb) | 10.855 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| Related compounds | |
Related alkanoic acids
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
N-Acetylaspartic acid, or N-acetylaspartate (NAA), is a derivative of aspartic acid with a formula of C6H9NO5 and a molecular weight of 175.139.
NAA is the second-most-concentrated molecule in the brain after the amino acid glutamate. It is detected in the adult brain in neurons,[2] oligodendrocytes and myelin[3] and is synthesized in the mitochondria from the amino acid aspartic acid and acetyl-coenzyme A.[4]
Function
The various functions served by NAA are under investigation, but the primary proposed functions include:
- Neuronal osmolyte that is involved in fluid balance in the brain
- Source of acetate for lipid and myelin synthesis in oligodendrocytes, the glial cells that myelinate neuronal axons
- Precursor for the synthesis of the neuronal dipeptide N-Acetylaspartylglutamate
- Contributor to energy production from the amino acid glutamate in neuronal mitochondria
In the brain, NAA was thought to be present predominantly in neuronal cell bodies, where it acts as a neuronal marker,[5] but it is also free to diffuse throughout neuronal fibers.[6]
Applications
However, the recent discovery of a higher concentration of NAA in myelin and oligodendrocytes than in neurons raises questions about the validity of the use of NAA as a neuronal marker.[3] NAA gives off the largest signal in magnetic resonance spectroscopy of the human brain. The levels measured there are decreased in numerous neuropathological conditions ranging from brain injury to stroke to Alzheimer's disease. This fact makes NAA a potential diagnostic molecule for doctors treating patients with brain damage or disease.
NAA may be a marker of creativity.[7] High NAA levels in the hippocampus are related to better working memory performance in humans.[8] Whole-brain levels of NAA have also been found to be positively correlated with educational attainment in adults.[9]
NAA may function as a neurotransmitter in the brain by acting on metabotropic glutamate receptors.[10]
See also
References
- ↑ "N-acetylaspartate - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=97508.
- ↑ "Immunocytochemical localization of N-acetyl-aspartate with monoclonal antibodies". Neuroscience 45 (1): 37–45. 1991. doi:10.1016/0306-4522(91)90101-s. PMID 1754068.
- ↑ 3.0 3.1 "Localisation of N-acetylaspartate in oligodendrocytes/myelin". Brain Structure & Function 220 (2): 899–917. March 2015. doi:10.1007/s00429-013-0691-7. PMID 24379086.
- ↑ "Synthesis of N-acetyl-L-aspartate by rat brain mitochondria and its involvement in mitochondrial/cytosolic carbon transport". The Biochemical Journal 184 (3): 539–46. December 1979. doi:10.1042/bj1840539. PMID 540047.
- ↑ "Nuclear magnetic resonance spectroscopy and imaging in animal research". ILAR Journal 42 (3): 189–208. 2001. doi:10.1093/ilar.42.3.189. PMID 11406719.
- ↑ "Brain intracellular metabolites are freely diffusing along cell fibers in grey and white matter, as measured by diffusion-weighted MR spectroscopy in the human brain at 7 T". Brain Structure & Function 221 (3): 1245–54. April 2016. doi:10.1007/s00429-014-0968-5. PMID 25520054.
- ↑ Geddes, Linda. "Creativity chemical favours the smart". https://www.newscientist.com/article/mg20227084.300-creativity-chemical-favours-the-smart.html.
- ↑ "Working memory and N-acetylaspartate level in hippocampus, parietal cortex and subventricular zone". International Journal of Psychology 47: 584. 2012. doi:10.1080/00207594.2012.709117.
- ↑ "The whole-brain N-acetylaspartate correlates with education in normal adults". Psychiatry Research: Neuroimaging 204 (1): 49-54. 30 October 2012. doi:10.1016/j.pscychresns.2012.04.013. PMC 3508436. https://doi.org/10.1016%2Fj.pscychresns.2012.04.013.
- ↑ "Activation by N-acetyl-L-aspartate of acutely dissociated hippocampal neurons in rats via metabotropic glutamate receptors". Epilepsia 44 (9): 1153–9. September 2003. doi:10.1046/j.1528-1157.2003.49402.x. PMID 12919386.
Further reading
- N-Acetylaspartate: A Unique Neuronal Molecule in the Central Nervous System. Springer Science & Business Media. 21 October 2006. ISBN 978-0-387-30172-3. https://books.google.com/books?id=tR44q6WWRBgC.
- "Biochemical support for the "threshold" theory of creativity: a magnetic resonance spectroscopy study". The Journal of Neuroscience 29 (16): 5319–25. April 2009. doi:10.1523/JNEUROSCI.0588-09.2009. PMID 19386928.
External links
 |

