Chemistry:Gamma-Amino-beta-hydroxybutyric acid
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Gamibetal, others |
| Other names | Buxamine; Buxamina; Bussamina; γ-Amino-β-hydroxybutyric acid; GABOB; β-Hydroxy-γ-aminobutyric acid; β-Hydroxy-GABA |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
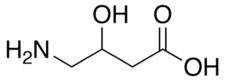
| Formula | C4H9NO3 |
| Molar mass | 119.120 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
γ-Amino-β-hydroxybutyric acid (GABOB), also known as β-hydroxy-γ-aminobutyric acid (β-hydroxy-GABA), and sold under the brand name Gamibetal among others, is an anticonvulsant which is used for the treatment of epilepsy in Europe, Japan , and Mexico.[1][2] It is a GABA analogue, or an analogue of the neurotransmitter γ-aminobutyric acid (GABA), and has been found to be an endogenous metabolite of GABA.[2][3][4][5]
Medical uses
GABOB is an anticonvulsant and is used in the treatment of epilepsy.[1][2]
Pharmacology
GABOB is a GABA receptor agonist.[6] It has two stereoisomers, and shows stereoselectivity in its actions.[6] Specifically, (R)-(–)-GABOB is a moderate-potency agonist of the GABAB receptor, while (S)-(+)-GABOB is a partial agonist of the GABAB receptor and an agonist of the GABAA receptor.[6] (S)-(+)-GABOB is around twice as potent an anticonvulsant as (R)-(–)-GABOB.[7] GABOB is used medically as a racemic mixture.[6]
Relative to GABA, GABOB has more potent inhibitory effects on the central nervous system, perhaps due to its greater capacity to cross the blood–brain barrier.[5][8] However, GABOB is of relatively low potency as an anticonvulsant when used by itself, and is more useful as an adjuvant treatment used alongside another anticonvulsant.[9][10]
Chemistry
GABOB, or β-hydroxy-GABA, is a close structural analogue of GABA (see GABA analogue), as well as of γ-hydroxybutyric acid (GHB), phenibut (β-phenyl-GABA), baclofen (β-(4-chlorophenyl)-GABA),[11] and pregabalin (β-isobutyl-GABA).
Society and culture
Generic name
GABOB has been referred to by the generic name buxamine or buxamina.[1][6]
Brand names
GABOB is sold primarily under the brand name Gamibetal.[1] It has also been marketed under a variety of other brand names including Aminoxan, Bogil, Diastal, Gabimex, Gabomade, Gaboril, Gamalate, and Kolpo.[1][12]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 44–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA44.
- ↑ 2.0 2.1 2.2 "Biologically Important Amino Acids". Methods of Non-α-Amino Acid Synthesis (Second ed.). CRC Press. 23 October 2013. pp. 146–. ISBN 978-1-4665-7789-3. https://books.google.com/books?id=pWxmAQAAQBAJ&pg=PA146.
- ↑ The Biochemical Basis of Neuropharmacology. Oxford University Press. 2003. pp. 112–. ISBN 978-0-19-514007-1. https://books.google.com/books?id=vNaM55VDoF8C&pg=PA112.
- ↑ "Dose-related effects of gamma-amino beta-hydroxy butyric acid (GABOB) infusion on growth hormone secretion in normal women". Journal of Endocrinological Investigation 5 (2): 101–106. 2014. doi:10.1007/BF03350499. PMID 7096918.
- ↑ 5.0 5.1 "The inhibitory action of beta-hydroxy-gamma-aminobutyric acid upon the seizure following stimulation of the motor cortex of the dog". The Journal of Physiology 145 (3): 570–578. March 1959. doi:10.1113/jphysiol.1959.sp006163. PMID 13642322.
- ↑ 6.0 6.1 6.2 6.3 6.4 "Chemistry of GABAB Receptor Ligands: Focus on Agonists and Antagonists". GABAB Receptor. Springer. 17 January 2017. pp. 25–. ISBN 978-3-319-46044-4. https://books.google.com/books?id=ONHBDQAAQBAJ&pg=PA25.
- ↑ "Different Efficacies of d- and l-γ-Amino-β-Hydroxybutyric Acids in GABA Receptor and Transport Test Systems". The Journal of Neuroscience 1 (2): 132–140. February 1981. doi:10.1523/JNEUROSCI.01-02-00132.1981. PMID 6267220.
- ↑ "Gamma-amino-beta-hydroxybutyric acid (GABOB) and brain serotonin". Psychopharmacologia 5 (1): 84–86. October 1963. doi:10.1007/BF00405577. PMID 14085623.
- ↑ "[Effect of gamma-amino-beta-hydroxybutyric acid (GABHB) on experimentally-induced epileptic activity]" (in it). Rivista di Neurologia 50 (4): 253–268. 1980. PMID 7466221.
- ↑ "gamma-Amino-beta-hydroxybutyric acid as add-on therapy in adult patients with severe focal epilepsy". Stereotactic and Functional Neurosurgery 69 (1-4 Pt 2): 243–246. 1997. doi:10.1159/000099882. PMID 9711762.
- ↑ "Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug". CNS Drug Reviews 7 (4): 471–481. 2001. doi:10.1111/j.1527-3458.2001.tb00211.x. PMID 11830761.
- ↑ European Drug Index: European Drug Registrations (Fourth ed.). CRC Press. 19 June 1998. pp. 181–. ISBN 978-3-7692-2114-5. https://books.google.com/books?id=2HBPHmclMWIC&pg=PA181.
{{Navbox | name = GABA receptor modulators | title = GABA receptor modulators | state = collapsed | bodyclass = hlist | groupstyle = text-align:center;
| group1 = Ionotropic | list1 = {{Navbox|subgroup | groupstyle = text-align:center | groupwidth = 5em
| group1 = GABAA | list1 =
- Agonists: (+)-Catechin
- Bamaluzole
- Barbiturates (e.g., phenobarbital)
- BL-1020
- DAVA
- Dihydromuscimol
- GABA
- Gabamide
- GABOB
- Gaboxadol (THIP)
- Homotaurine (tramiprosate, 3-APS)
- Ibotenic acid
- iso-THAZ
- iso-THIP
- Isoguvacine
- Isomuscimol
- Isonipecotic acid
- Kojic amine
- Lignans (e.g., honokiol)
- Methylglyoxal
- Monastrol
- Muscimol
- Nefiracetam
- Neuroactive steroids (e.g., allopregnanolone)
- Org 20599
- PF-6372865
- Phenibut
- Picamilon
- P4S
- Progabide
- Propofol
- Quisqualamine
- SL-75102
- TACA
- TAMP
- Terpenoids (e.g., borneol)
- Thiomuscimol
- Tolgabide
- ZAPA
- Positive modulators (abridged; see here for a full list): α-EMTBL
- Alcohols (e.g., ethanol)
- Anabolic steroids
- Avermectins (e.g., ivermectin)
- Barbiturates (e.g., phenobarbital)
- Benzodiazepines (e.g., diazepam)
- Bromide compounds (e.g., potassium bromide)
- Carbamates (e.g., meprobamate)
- Carbamazepine
- Chloralose
- Chlormezanone
- Clomethiazole
- Dihydroergolines (e.g., ergoloid (dihydroergotoxine))
- Etazepine
- Etifoxine
- Fenamates (e.g., mefenamic acid)
- Flavonoids (e.g., apigenin, hispidulin)
- Fluoxetine
- Flupirtine
- Imidazoles (e.g., etomidate)
- Kava constituents (e.g., kavain)<!--PMID: 9776662-->
- Lanthanum
- Loreclezole
- Monastrol
- Neuroactive steroids (e.g., allopregnanolone, [[Chemistry:Cholecholesterol]], THDOC)
- Niacin
- Nicotinamide (niacinamide)
- Nonbenzodiazepines (e.g., β-carbolines (e.g., [[abecarnil), cyclopyrrolones (e.g., zopiclone), imidazopyridines (e.g., zolpidem), pyrazolopyrimidines (e.g., zaleplon))
- Norfluoxetine
- Petrichloral
- Phenols (e.g., propofol)
- Phenytoin
- Piperidinediones (e.g., glutethimide)
- Propanidid
- Pyrazolopyridines (e.g., etazolate)
- Quinazolinones (e.g., methaqualone)
- Retigabine (ezogabine)
- ROD-188
- Skullcap constituents (e.g., baicalin)
- Stiripentol
- Sulfonylalkanes (e.g., sulfonmethane (sulfonal))
- Topiramate
- Valerian constituents (e.g., valerenic acid)
- Volatiles/gases (e.g., chloral hydrate, chloroform, [[Chemistry:Diethyl diethyl ether, Parparaldehyde]], sevoflurane)
- Antagonists: Bicuculline
- Coriamyrtin
- Dihydrosecurinine
- Gabazine (SR-95531)
- Hydrastine
- Hyenachin (mellitoxin)
- PHP-501
- Pitrazepin
- Securinine
- Sinomenine
- SR-42641
- SR-95103
- Thiocolchicoside
- Tutin
- Negative modulators: 1,3M1B
- 3M2B
- 11-Ketoprogesterone
- 17-Phenylandrostenol
- α5IA (LS-193,268)
- β-CCB
- β-CCE
- β-CCM
- β-CCP
- β-EMGBL
- Anabolic steroids
- Amiloride
- Anisatin
- β-Lactams (e.g., penicillins, cephalosporins, carbapenems)
- Basmisanil
- Bemegride
- Bicyclic phosphates (TBPS, TBPO, IPTBO)
- BIDN
- Bilobalide
- Bupropion
- CHEB
- Chlorophenylsilatrane
- Cicutoxin
- Cloflubicyne
- Cyclothiazide
- DHEA
- DHEA-S
- Dieldrin
- (+)-DMBB
- DMCM
- DMPC
- EBOB
- Etbicyphat
- FG-7142 (ZK-31906)
- Fiproles (e.g., fipronil)
- Flavonoids (e.g., amentoflavone, oroxylin A)
- Flumazenil
- Fluoroquinolones (e.g., ciprofloxacin)
- Flurothyl
- Furosemide
- Golexanolone
- Iomazenil (123I)
- IPTBO
- Isopregnanolone (sepranolone)
- L-655,708
- Laudanosine
- Leptazol
- Lindane
- MaxiPost
- Morphine
- Morphine-3-glucuronide
- MRK-016
- Naloxone
- Naltrexone
- Nicardipine
- Nonsteroidal antiandrogens (e.g., [[apalutamide, [[Chemistry:Bicalutbicalutamide, Enzalutenzalutamide, Chemistry:Flutamide|flut]]amide]], nilutamide)
- Oenanthotoxin
- Pentylenetetrazol (pentetrazol)
- Phenylsilatrane
- Picrotoxin (i.e., picrotin, picrotoxinin and dihydropicrotoxinin)
- Pregnenolone sulfate
- Propybicyphat
- PWZ-029
- Radequinil
- Ro 15-4513
- Ro 19-4603
- RO4882224
- RO4938581
- Sarmazenil
- SCS
- Suritozole
- TB-21007
- TBOB
- TBPS
- TCS-1105
- Terbequinil
- TETS
- Thujone
- U-93631
- Zinc
- ZK-93426
| group2 = GABAA-ρ | list2 =
- Agonists: BL-1020
- CACA
- CAMP
- Homohypotaurine
- GABA
- GABOB
- Ibotenic acid
- Isoguvacine
- Muscimol
- N4-Chloroacetylcytosine arabinoside
- Picamilon
- Progabide
- TACA
- TAMP
- Thiomuscimol
- Tolgabide
- Positive modulators: Allopregnanolone
- Alphaxolone
- ATHDOC
- Lanthanides
- Antagonists: (S)-2-MeGABA
- (S)-4-ACPBPA
- (S)-4-ACPCA
- 2-MeTACA
- 3-APMPA
- 4-ACPAM
- 4-GBA
- cis-3-ACPBPA
- CGP-36742 (SGS-742)
- DAVA
- Gabazine (SR-95531)
- Gaboxadol (THIP)
- I4AA
- Isonipecotic acid
- Loreclezole
- P4MPA
- P4S
- SKF-97541
- SR-95318
- SR-95813
- TPMPA
- trans-3-ACPBPA
- ZAPA
- Negative modulators: 5α-Dihydroprogesterone
- Bilobalide
- Loreclezole
- Picrotoxin (picrotin, picrotoxinin)
- Pregnanolone
- ROD-188
- THDOC
- Zinc
}}
| group2 = Metabotropic
| list2 =
