Chemistry:Cingestol
From HandWiki
Short description: Chemical compound
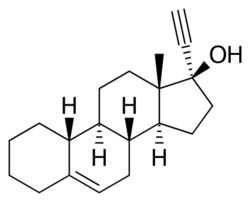 | |
| Clinical data | |
|---|---|
| Trade names | Lutisan |
| Other names | 19-Nor-17α-pregn-5-en-20-yn-17β-ol; O.V. 28[1][2] |
| Routes of administration | Oral |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H28O |
| Molar mass | 284.443 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cingestol (INN, USAN) (former tentative brand name Lutisan),[3] also known as 17α-ethynylestr-5-en-17β-ol,[4] is a steroidal progestin of the 19-nortestosterone group[5][6] that was never marketed.[7] It was synthesized in 1969[7] and was developed in the 1970s by Organon as a low-dose, progestogen-only contraceptive,[8][9][10][11] but in 1984, was still described as "under investigation".[12] The drug is an isomer of lynestrenol with the double bond between C5 and C6.[1]
See also
References
- ↑ 1.0 1.1 Pharmacology of Hormones. Thieme. 1975. p. 126,129. ISBN 978-3-13-518901-7. https://books.google.com/books?id=9WhNAQAAIAAJ.
- ↑ Concours médical. 1976. p. 1083. https://books.google.com/books?id=_UQgAQAAMAAJ.
- ↑ Registry of Toxic Effects of Chemical Substances. National Institute for Occupational Safety and Health. 1987. p. 2995. https://books.google.com/books?id=dRFV2ZV6DT0C.
- ↑ Sex hormone pharmacology. Academic Press. 1976. p. 12. ISBN 978-0-12-137250-7. https://books.google.com/books?id=zt5sAAAAMAAJ.
- ↑ Essentials of Medicinal Chemistry. Wiley. 16 August 1988. ISBN 978-0-471-88356-2. https://books.google.com/books?id=6hxtAAAAMAAJ.
- ↑ WHO Technical Report Series. World Health Organization. 1981. p. 75. ISBN 9789241206570. https://books.google.com/books?id=WlQYAAAAMAAJ.
- ↑ 7.0 7.1 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 279–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA279.
- ↑ Directory of selected scholars and researchers in Southeast Asia. Regional Institute of Higher Education and Development. 1974. p. 687. https://books.google.com/books?id=mB4gAQAAMAAJ.
- ↑ Biochemical contraception: prospects for human development. Academic Press. 1976. p. 283. ISBN 978-0-12-134640-9. https://books.google.com/books?id=7pRsAAAAMAAJ.
- ↑ "Effect of minipills on physiologic responses of human cervical mucus, endometrium, and ovary". Fertility and Sterility 24 (8): 578–583. August 1973. doi:10.1016/s0015-0282(16)39850-8. PMID 4124151.
- ↑ "Clinical evaluation of a new low dose progestagen-only contraceptive containing cingestol". International Journal of Fertility 19 (3): 171–175. 1974. PMID 4375129. http://www.popline.org/node/494146.
- ↑ Cutting's Handbook of Pharmacology: The Actions and Uses of Drugs. Appleton-Century-Crofts. 1984. p. 439. ISBN 978-0-8385-1418-4. https://books.google.com/books?id=B-1sAAAAMAAJ.
 |
