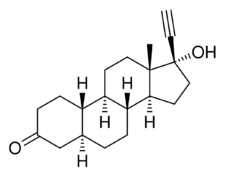
Chemistry:5α-Dihydronorethisterone
 | |
| Clinical data | |
|---|---|
| Other names | 5α-DHNET; 5α-Dihydro-NET; Dihydronorethisterone; Dihydronorethindrone; DHNET; 17α-Ethynyl-5α-dihydro-19-nortestosterone; 17α-Ethynyl-5α-estran-17β-ol-3-one; STS-737; NSC-85401; 19-Nor-5α,17α-pregn-20-yn-17-ol-3-one |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C20H28O2 |
| Molar mass | 300.442 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
5α-Dihydronorethisterone (5α-DHNET, dihydronorethisterone, 17α-ethynyl-5α-dihydro-19-nortestosterone, or 17α-ethynyl-5α-estran-17β-ol-3-one) is a major active metabolite of norethisterone (norethindrone).[1][2][3][4] Norethisterone is a progestin with additional weak androgenic and estrogenic activity.[1] 5α-DHNET is formed from norethisterone by 5α-reductase in the liver and other tissues.[1][2][3][4]
Pharmacology
Unlike norethisterone which is purely progestogenic, 5α-DHNET has been found to possess both progestogenic and marked antiprogestogenic activity, showing a profile of progestogenic activity like that of a selective progesterone receptor modulator (SPRM).[4] Moreover, the affinity of 5α-DHNET for the progesterone receptor (PR) is greatly reduced relative to that of norethisterone at only 25% of that of progesterone (versus 150% for norethisterone).[1]
5α-DHNET shows higher affinity for the androgen receptor (AR) compared to norethisterone with approximately 27% of the affinity of the potent androgen metribolone (versus 15% for norethisterone).[1] However, although 5α-DHNET has higher affinity for the AR than does norethisterone, it has significantly diminished and in fact almost abolished androgenic activity in comparison to norethisterone in rodent bioassays.[2][5] Similar findings were observed for ethisterone (17α-ethynyltestosterone) and its 5α-reduced metabolite, whereas 5α-reduction enhanced both the AR affinity and androgenic potency of testosterone and nandrolone (19-nortestosterone) in rodent bioassays.[5] As such, it appears that the C17α ethynyl group of norethisterone is responsible for its loss of androgenicity upon 5α-reduction.[5] Instead of androgenic activity, 5α-DHNET has been reported to possess some antiandrogenic activity.[6]
Norethisterone and 5α-DHNET have been found to act as weak irreversible aromatase inhibitors (Ki = 1.7 μM and 9.0 μM, respectively).[7] However, the concentrations required are probably too high to be clinically relevant at typical dosages of norethisterone.[1] 5α-DHNET specifically has been assessed and found to be selective in its inhibition of aromatase, and does not affect other steroidogenesis enzymes such as cholesterol side-chain cleavage enzyme (P450scc), 17α-hydroxylase/17,20-lyase, 21-hydroxylase, or 11β-hydroxylase.[7] Since it is not aromatized (and hence cannot be transformed into an estrogenic metabolite), unlike norethisterone, 5α-DHNET has been proposed as a potential therapeutic agent in the treatment of estrogen receptor (ER)-positive breast cancer.[7]
| Compound | Typea | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|---|
| Norethisterone | – | 67–75 | 15 | 0 | 0–1 | 0–3 | 16 | 0 |
| 5α-Dihydronorethisterone | Metabolite | 25 | 27 | 0 | 0 | ? | ? | ? |
| 3α,5α-Tetrahydronorethisterone | Metabolite | 1 | 0 | 0–1 | 0 | ? | ? | ? |
| 3α,5β-Tetrahydronorethisterone | Metabolite | ? | 0 | 0 | ? | ? | ? | ? |
| 3β,5α-Tetrahydronorethisterone | Metabolite | 1 | 0 | 0–8 | 0 | ? | ? | ? |
| Ethinylestradiol | Metabolite | 15–25 | 1–3 | 112 | 1–3 | 0 | 0.18 | 0 |
| Norethisterone acetate | Prodrug | 20 | 5 | 1 | 0 | 0 | ? | ? |
| Norethisterone enanthate | Prodrug | ? | ? | ? | ? | ? | ? | ? |
| Noretynodrel | Prodrug | 6 | 0 | 2 | 0 | 0 | 0 | 0 |
| Etynodiol | Prodrug | 1 | 0 | 11–18 | 0 | ? | ? | ? |
| Etynodiol diacetate | Prodrug | 1 | 0 | 0 | 0 | 0 | ? | ? |
| Lynestrenol | Prodrug | 1 | 1 | 3 | 0 | 0 | ? | ? |
| Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone for the AR, estradiol for the ER, dexamethasone for the GR, aldosterone]] for the MR, dihydrotestosterone for SHBG, and cortisol for CBG. Footnotes: a = Active]] or inactive metabolite, prodrug, or neither of norethisterone. Sources: See template. | ||||||||
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric 8 (Suppl 1): 3–63. August 2005. doi:10.1080/13697130500148875. PMID 16112947.
- ↑ 2.0 2.1 2.2 "Structural characteristics of anabolic androgenic steroids contributing to binding to the androgen receptor and to their anabolic and androgenic activities. Applied modifications in the steroidal structure". Steroids 74 (2): 172–197. February 2009. doi:10.1016/j.steroids.2008.10.016. PMID 19028512. "Many synthetic steroids with high myotrophic activity exhibit myotrophic–androgenic dissociation, since, due to changes introduced in the structure of ring A, they will probably not be substrates for the 5α-reductases [85]. 5α-Reduction does not always amplify the androgenic potency in spite of high RBA of androgens to the AR. This is the case for norethisterone (Fig. 1, 34), a synthetic 19-nor-17α-ethynyl testosterone derivative, which also undergoes enzyme-mediated 5α-reduction and exerts potent androgenic effects in target organs. 5α-Reduced norethisterone displays a higher AR binding but shows a significantly lower androgenic potency than unchanged norethisterone [102,103].".
- ↑ 3.0 3.1 "[Effect of synthetic analogs of the sex steroid hormones on the secretory and proliferative activity of the adenohypophysis in vivo and in vitro in rats]" (in ru). Farmakologiia I Toksikologiia 51 (5): 57–61. 1988. PMID 3208885.
- ↑ 4.0 4.1 4.2 "[Antiprogestational action of 5 alpha-dihydronorethisterone]" (in zh). Zhongguo Yao Li Xue Bao = Acta Pharmacologica Sinica 6 (2): 125–129. June 1985. PMID 2934946.
- ↑ 5.0 5.1 5.2 "5alpha-reduction of norethisterone enhances its binding affinity for androgen receptors but diminishes its androgenic potency". The Journal of Steroid Biochemistry and Molecular Biology 60 (1–2): 121–129. January 1997. doi:10.1016/s0960-0760(96)00172-0. PMID 9182866.
- ↑ "Progress towards hormonal male contraception". Trends in Pharmacological Sciences 25 (1): 49–57. January 2004. doi:10.1016/j.tips.2003.11.009. PMID 14723979.
- ↑ 7.0 7.1 7.2 "Suicide inactivation of aromatase in human placenta and uterine leiomyoma by 5 alpha-dihydronorethindrone, a metabolite of norethindrone, and its effect on steroid-producing enzymes". European Journal of Endocrinology 130 (6): 634–640. June 1994. doi:10.1530/eje.0.1300634. PMID 8205267.
 |

