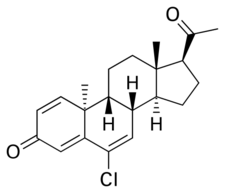
Chemistry:Trengestone
 | |
| Clinical data | |
|---|---|
| Trade names | Reteroid, Retroid, Retrone |
| Other names | Ro 4-8347; Triengestone; 1,6-Didehydro-6-chlororetroprogesterone; 6-Chloro-9β-10α-pregna-1,4,6-triene-3,20-dione |
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ≥41–46% (based on urinary excretion)[1] |
| Metabolism | Liver[2][3] |
| Metabolites | • 20α-Dihydrotrengestone[1] |
| Elimination half-life | • Trengestone: very short[1] • 20α-DHTG: 8–14 hours[1] |
| Excretion | Urine: 41–46%[1] Feces: 30% (unchanged)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H25ClO2 |
| Molar mass | 344.88 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Trengestone, sold under the brand names Reteroid, Retroid, and Retrone, is a progestin medication which was formerly used to treat menstrual disorders but is now no longer marketed.[4][5][6][7][8] It is taken by mouth.[9]
Side effects of trengestone include headache, fatigue, and breast tenderness among others.[7] Trengestone is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[7] It is not androgenic or estrogenic.[7]
Trengestone was introduced for medical use in 1974.[5] It is no longer available.[8]
Medical uses
Trengestone was used in the treatment of menstrual disorders.[8] It has also been used to induce ovulation, with about a 50% success rate on average.[7]
Side effects
Side effects of trengestone include headache, fatigue, and breast tenderness among others.[7] It is not androgenic and does not cause masculinization.[7]
Pharmacology
Pharmacodynamics
Trengestone is a progestogen, or an agonist of the progesterone receptor.[7] It is an atypical progestogen similarly to dydrogesterone.[7] For instance, unlike other progestogens, trengestone and dydrogesterone do not increase body temperature (i.e., have no hyperthermic effect).[7][10][11] In addition, whereas other progestogens are antigonadotropic and inhibit ovulation, dydrogesterone is neither antigonadotropic nor progonadotropic and does not affect ovulation, and trengestone appears to be progonadotropic and can be used to induce ovulation.[7][11][12] Similarly to dydrogesterone and progesterone, trengestone has no androgenic or estrogenic activity.[7][11]
Pharmacokinetics
Trengestone appears to be a prodrug of 20α-dihydrotrengestone (20α-DHTG), as it is largely transformed into this major metabolite upon oral administration.[1][13] 20α-DHTG has potent progestogenic activity, with peak levels of this metabolite occurring at 2 to 4 hours following administration of trengestone and with a biological half-life of 8 to 14 hours.[1] Trengestone is excreted 41 to 46% in urine and up to 30% unchanged in feces, suggesting that a significant portion of the medication is not absorbed from the gastrointestinal tract.[1] The metabolism and pharmacokinetics of trengestone have been reviewed.[2][3]
Chemistry
Trengestone, also known as 1,6-didehydro-6-chlororetroprogesterone or as 6-chloro-9β,10α-pregna-1,4,6-triene-3,20-dione, is a synthetic pregnane steroid and a derivative of progesterone and retroprogesterone.[4][7][14] Retroprogesterone derivatives like trengestone are analogues of progesterone in which the hydrogen atom at the 9th carbon has been switched from the α-position (below the plane) to the β-position (above the plane) and the methyl group at the 10th carbon has been switched from the β-position to the α-position.[7] This results in a "bent" configuration in which the plane of rings A and B is orientated at a 60° angle below the rings C and D.[11] Analogues of trengestone include dydrogesterone (6-dehydroretroprogesterone) and Ro 6-3129 (16α-ethylthio-6-dehydroretroprogesterone).[4]
History
Trengestone was synthesized in 1964 and was introduced for medical use by Roche in 1974.[4][5][6]
Society and culture
Generic names
Trengestone is the generic name of the drug and its INN.[4][6] It is also known by its former developmental code name Ro 4-8347.[4][6]
Brand names
Trengestone was marketed under the brand names Reteroid, Retroid, and Retrone.[4]
Availability
Trengestone is no longer marketed and hence is no longer available in any country.[8]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 "Disposition of the retro-steroid progestogen, 6-chloro-9beta, 10alpha-pregna-1,4,6-triene-3,20-dione (Ro 4-8347), in man". Contraception 11 (3): 339–346. March 1975. doi:10.1016/0010-7824(75)90042-6. PMID 1116370.
- ↑ 2.0 2.1 "The metabolism of the synthetic progestational compound Ro 4-8347". Bulletin der Schweizerischen Akademie der Medizinischen Wissenschaften 25 (4–6): 337–348. October 1970. PMID 5510163. https://www.popline.org/node/475459. Retrieved 2018-03-01.
- ↑ 3.0 3.1 "Metabolism of progesterone and synthetic progestational agents". Bulletin der Schweizerischen Akademie der Medizinischen Wissenschaften 25 (4–6): 300–315. October 1970. PMID 5510160. https://www.popline.org/node/474775. Retrieved 2018-03-01.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 "6-Chloro-9β-10α-pregna-1,4,6-triene-3,20-dione". The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 259–. C-00276. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA259.
- ↑ 5.0 5.1 5.2 Médicaments pour tous en l'an 2000?: les multinationales pharmaceutiques suisses face au tiers monde : l'exemple du Mexique. Editions d'en bas. 1983. pp. 93–. ISBN 978-2-8290-0039-3. https://books.google.com/books?id=ZgLwphUSEN0C&pg=PA93.
- ↑ 6.0 6.1 6.2 6.3 Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 31 October 1999. pp. 279–. ISBN 978-0-7514-0499-9. https://books.google.com/books?id=mqaOMOtk61IC&pg=PA279.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 "Therapy of Anovulation". Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. 6 December 2012. pp. 329–. ISBN 978-94-009-8195-9. https://books.google.com/books?id=7IrpCAAAQBAJ&pg=PA329.
- ↑ 8.0 8.1 8.2 8.3 "Micromedex". Merative US L.P.. http://www.micromedexsolutions.com.
- ↑ "Chapter 17. Steroids and Biologically Related Compounds". Annual Reports in Medicinal Chemistry. 6. Academic Press. 1971. pp. 162–181. doi:10.1016/S0065-7743(08)60972-0. ISBN 9780120405060. "Ro 4-8347 (21), a potent orally active progestagen, when given at the dose of 4 mg/day in the second half of the cycle, was found clinically useful in anovulatory women with decreased ovarian function.109"
- ↑ "Luteal phase insufficiency". Contributions to Gynecology and Obstetrics 4: 78–113. 1978. doi:10.1159/000401245. ISBN 978-3-8055-2791-0. PMID 679688. "Fig. 17. Lack of hyperthermic effect of retroprogesterone derivative (Trengestone).".
- ↑ 11.0 11.1 11.2 11.3 "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric 8 (Suppl 1): 3–63. August 2005. doi:10.1080/13697130500148875. PMID 16112947.
- ↑ Hormonal Steroids: Proceedings of the Third International Congress on Hormonal Steroids, Hamburg, 7-12 September 1970. 3. Excerpta Medica. 1971. pp. 873–874, 876. ISBN 978-90-219-0144-2. https://books.google.com/books?id=2ZkKAQAAMAAJ. "Trengestone, contrary to [dydrogesterone], not only does not inhibit ovarian activity while exerting a progestation effect, but it stimulates the former. One tablet per day is administered from the 5th [...] Both dydrogesterone and trengestone can inhibit ovulation in the rat and rabbit, but only the latter compound can do so in women — at doses far above the therapeutic range. Various clinical reports have suggested, on the basis of quite unrelated findings, that trengestone may, despite lack of inherent estrogenicity, somehow cause an indirect stimulation of the production of endogenous estrogens. Numerous investigators (Stamm et al., 1968; Dapunt and Windbichler, 1970) have satisfied themselves that the compound may stimulate ovulation in women with certain endocrinologic imbalances or deficiencies [...]"
- ↑ "Metabolism of 6-chloro-9 beta, 10 alpha-pregna-1,4,6-triene-3,20-dione in rat, rabbit, monkey and man". Acta Endocrinologica 74 (1): 127–143. September 1973. doi:10.1530/acta.0.0740127. PMID 4202495.
- ↑ "[Clinical studies and endometrial histomorphological findings by means of a new retroprogesterone derivative: 1,6 bis dehydro-6-chloro-retroprogesterone (Trengestone)]" (in it). Minerva Ginecologica 22 (18): 874–879. September 1970. PMID 4925556.
 |
