Chemistry:Mespirenone
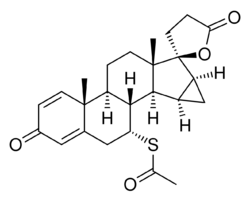 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C25H30O4S |
| Molar mass | 426.57 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Mespirenone (INN) (developmental code name ZK-94679), also known as Δ1-15β,16β-methylenespironolactone, is a steroidal antimineralocorticoid of the spirolactone group related to spironolactone that was never marketed.[1][2] Animal research found that it was 3.3-fold more potent as an antimineralocorticoid relative to spironolactone.[3] In addition to its antimineralocorticoid properties, mespirenone is also a progestogen, antigonadotropin, and antiandrogen.[2][4] It is 2- to 3-fold as potent as spironolactone as a progestogen and antigonadotropin but its antiandrogenic activity is markedly reduced and weak (though still of significance) in comparison.[4][5] Mespirenone is also a potent and specific enzyme inhibitor of 18-hydroxylase and thus of mineralocorticoid biosynthesis.[6] The drug was under development by Schering (now Bayer Schering Pharma) and reached phase II clinical trials but was discontinued in 1989.[7]
See also
References
- ↑ The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 775–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA775.
- ↑ 2.0 2.1 "Mespirenone and other 15,16-methylene-17-spirolactones, a new type of steroidal aldosterone antagonists". Arzneimittel-Forschung 36 (11): 1583–1600. November 1986. PMID 3028435.
- ↑ "Interference of C17-spirosteroids with late steps of aldosterone biosynthesis. Structure-activity studies". Arzneimittel-Forschung 41 (10): 1082–1091 (1083). October 1991. PMID 1799390. https://books.google.com/books?id=QtcTAQAAMAAJ.
- ↑ 4.0 4.1 "Experimental studies on the endocrine side effects of new aldosterone antagonists". Arzneimittel-Forschung 38 (12): 1800–1805. December 1988. PMID 3245852.
- ↑ "Effect of a new mineralocorticoid antagonist mespirenone on aldosterone-induced hypertension". The American Journal of Physiology 260 (2 Pt 1): E269–E271. February 1991. doi:10.1152/ajpendo.1991.260.2.E269. PMID 1996630.
- ↑ "Inhibitory effects of the novel anti-aldosterone compound mespirenone on adrenocortical steroidogenesis in vitro". Arzneimittel-Forschung 41 (9): 946–949. September 1991. PMID 1796922.
- ↑ "Nuclear receptors as targets in cardiovascular diseases". Nuclear Receptors as Drug Targets. John Wiley & Sons. 8 September 2008. pp. 410–. ISBN 978-3-527-62330-3. https://books.google.com/books?id=iATfLbPgRugC&pg=PA410.
 |
