Chemistry:Hydroxyprogesterone heptanoate
 | |
| Clinical data | |
|---|---|
| Trade names | H.O.P, Hydroxyprogesterone, Lutogil A.P., Lutogyl A.P., others |
| Other names | OHPH; Hydroxyprogesterone enanthate; OHPE; 17α-Hydroxyprogesterone heptanoate; 17α-Hydroxyprogesterone heptylate; 17α-Hydroxypregn-4-ene-3,20-dione 17α-heptanoate; 17α-Heptyloylpregn-4-ene-3,20-dione |
| Routes of administration | Intramuscular injection |
| Drug class | Progestogen; Progestin; Progestogen ester |
| ATC code | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C28H42O4 |
| Molar mass | 442.640 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Hydroxyprogesterone heptanoate (OHPH), also known as hydroxyprogesterone enanthate (OHPE) and sold under the brand names H.O.P., Lutogil A.P., and Lutogyl A.P. among others, is a progestin medication used for progestogenic indications.[1][2][3][4] It has been formulated both alone and in together with estrogens, androgens/anabolic steroids, and other progestogens in several combination preparations (brand names Tocogestan, Trioestrine Retard, and Triormon Depositum).[4][5][6][7][8][9][10] OHPH is given by injection into muscle at regular intervals.[11][9]
OHPH is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[12][13][14] It appears to have similar pharmacology to that of the closely related medication hydroxyprogesterone caproate (OHPC).[15][16][17]
OHPH was first described by 1954[16] and was introduced for medical use by 1957.[6] It has been used clinically in France and Monaco in the past but is no longer marketed.[2][3][4]
Medical uses
OHPH is a progestogen and was used in situations in which progestogens were indicated.[12][13][14]
Available forms
OHPH was provided as a 125 mg/1 mL oil solution for use by intramuscular injection.[3][11] In addition to single-drug preparations, OHPH has also been used in a number of multi-drug formulations.[4][5][6][7][8][9][10] It was used in Tocogestan, a combination of 50 mg progesterone, 200 mg OHPH, and 250 mg α-tocopherol palmitate (vitamin E) in oil solution for use by intramuscular injection.[18][4][5] It was also used in Triormon Depositum (estradiol dibutyrate, testosterone caproate, and OHPH) and Trioestrine Retard (estradiol diundecylate, testosterone cyclohexylpropionate, and OHPH).[6][7] OHPH was a component of the experimental preparation Trophobolene (or Trophoboline), which also contained estrapronicate (estradiol nicotinate propionate) and nandrolone undecanoate, as well.[8][9][10]
Pharmacology
Pharmacodynamics
OHPH is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[15][12][13][14] The progestogenic potency of OHPH in the uterus is equal to or greater than that of progesterone when administered by subcutaneous injection in animals.[15][16][17] Its potency in animals likewise appears to be similar to that of hydroxyprogesterone caproate.[15][16][17]
Pharmacokinetics
OHPH shows a pronounced depot effect when administered by subcutaneous injection in animals, similarly to the closely related medication hydroxyprogesterone caproate.[15][16] The oral activity of OHPH in animals does not appear to have been assessed.[15]
Chemistry
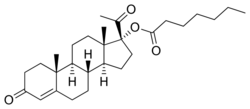
OHPH, also known as hydroxyprogesterone enanthate (OHPE),[19] as well as 17α-hydroxyprogesterone heptanoate or 17α-hydroxypregn-4-ene-3,20-dione 17α-heptanoate, is a synthetic pregnane steroid and a derivative of progesterone and 17α-hydroxyprogesterone.[1][2] It is a progestogen ester; specifically, it is the C17α heptanoate (enanthate) ester of 17α-hydroxyprogesterone.[1][2] Analogues of OHPH include the more well-known medications hydroxyprogesterone acetate and hydroxyprogesterone caproate (hydroxyprogesterone hexanoate).[1][2] The C3 benzilic acid hydrazone of OHPH, hydroxyprogesterone heptanoate benzilic acid hydrazone (OHPHBH), is known and has been studied in animals.[20][21] In terms of chemical structure, OHPH is very similar to hydroxyprogesterone caproate, differing from it only in having one additional carbon in its fatty acid ester chain.[1][2]
History
OHPH was first described, along with hydroxyprogesterone caproate and hydroxyprogesterone acetate, by Karl Junkmann of Schering AG in 1954.[16][19] It was introduced for medical use by 1957.[6] OHPH was commercialized by Roussel and Théramex, and has been used clinically in France and Monaco but is no longer marketed.[2][3][4]
Society and culture
Brand names
OHPH has been marketed alone under a number of brand names including H.O.P, Hydroxyprogesterone, Lutogil A.P., and Lutogyl A.P.[1][2][3][4]
Availability
OHPH was previously marketed in France and Monaco but is no longer available.[2][3][22]
See also
- Estradiol dibutyrate/hydroxyprogesterone heptanoate/testosterone caproate
- Estradiol diundecylate/hydroxyprogesterone heptanoate/testosterone cyclohexylpropionate
- Estrapronicate/hydroxyprogesterone heptanoate/nandrolone undecanoate
- Progesterone/hydroxyprogesterone heptanoate/α-tocopherol palmitate
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 665–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA665.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 532–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA532.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 European Drug Index: European Drug Registrations (Fourth ed.). CRC Press. 19 June 1998. pp. 612–. ISBN 978-3-7692-2114-5. https://books.google.com/books?id=2HBPHmclMWIC&pg=PA612.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Pharmaceutical Substances: Syntheses, Patents, Applications. Thieme. 2001. p. 1033. ISBN 978-3-13-558404-1. https://books.google.com/books?id=ym5qAAAAMAAJ.
- ↑ 5.0 5.1 5.2 "Neonatal neuroblastoma and in utero exposure to progestagens". International Journal of Risk and Safety in Medicine 11 (2): 121–128. 1998. https://content.iospress.com/articles/international-journal-of-risk-and-safety-in-medicine/jrs132.
- ↑ 6.0 6.1 6.2 6.3 6.4 "Triormon depositum in climacteric syndrome. Curves of excretion of catabolites and duration of the therapeutic effect". Quaderni Clin. Ostet. E Ginecol. 12: 284–93. 1957. "Triormon depositum (estradiol dibutyrate 3, testosterone caprylate 50, and hydroxyprogesterone heptanoate 30 mg.), administered in castor oil-benzyl benzoate soln. or polyvinylpyrrolidone suspension to 21 women in climacteric, was followed by estradiol, pregnanediol, and 17-keto steroid urinary curves, most with a peak at the 4th day, and approaching starting values at the 8-10th day. The therapeutic efficacy of the drug was satisfactory.".
- ↑ 7.0 7.1 7.2 "Cure of fifteen osteoporosis cases by a delayed effect of hormonal association". Semaine des Hopitaux 39 (2): 81–4. 1963. ISSN 0037-1777. "The patients (females) received intramuscularly, every 10 days for 2-3 months, estradiol diundecyleate 2.25, testosterone cyclohexylpropionate 67.5, and hydroxyprogesterone heptylate 100 mg. ("trioestrine retard"). Their av. calcuria decreased 30.5% (0-69%) and asthenia, anorexia, and muscular activity improved.".
- ↑ 8.0 8.1 8.2 Excerpta medica. Section 8, Neurology and neurosurgery. 1981. p. 10. https://books.google.com/books?id=_qiaAAAAIAAJ.
- ↑ 9.0 9.1 9.2 9.3 "Nandarolone". Testosterone Congeners—Advances in Research and Application: 2013 Edition: ScholarlyBrief. ScholarlyEditions. 21 June 2013. pp. 137–. ISBN 978-1-4816-9288-5. https://books.google.com/books?id=CR-NH3HzlSkC&pg=PA137.
- ↑ 10.0 10.1 10.2 Chromatography in Biochemistry, Medicine and Environmental Research: Proceedings of the ... International Symposium on Chromatography in Biochemistry, Medicine and Environmental Research. Elsevier Scientific Publishing Company. 1981. p. 99. ISBN 9780444420169. https://books.google.com/books?id=bbTwAAAAMAAJ.
- ↑ 11.0 11.1 Extended Release Dosage Forms. CRC Press. 1987. p. 12. ISBN 978-0-8493-4307-0. https://books.google.com/books?id=tCxtAAAAMAAJ. "Progestogens. [...] Hydroxyprogesterone heptanoate. Hydroxyprogesterone (Theramex). Oily solution for injection."
- ↑ 12.0 12.1 12.2 "The "newer" progestogens and postmenopausal hormone therapy (HRT)". The Journal of Steroid Biochemistry and Molecular Biology 142: 48–51. July 2014. doi:10.1016/j.jsbmb.2013.12.003. PMID 24333799.
- ↑ 13.0 13.1 13.2 "Progestogens in menopausal hormone therapy". Przeglad Menopauzalny = Menopause Review 14 (2): 134–143. June 2015. doi:10.5114/pm.2015.52154. PMID 26327902.
- ↑ 14.0 14.1 14.2 "Progesterone for the luteal support of assisted reproductive technologies: clinical options". Human Reproduction 15 (suppl 1): 129–148. June 2000. doi:10.1093/humrep/15.suppl_1.129. PMID 10928425.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 "Besonderheiten der Wirkungen der einzelnen Gestagene auf Morphologie und Funktion des Genitaltraktes bei Säugetieren". Die Gestagene. 2. Springer-Verlag. 1969. pp. 50–131. ISBN 978-3-662-00826-3. https://books.google.com/books?id=7kLPBgAAQBAJ&pg=PA69.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 "Über protrahiert wirksame Gestagene". Naunyn-Schmiedebergs Archiv für Experimentelle Pathologie und Pharmakologie 223 (3). 1954. doi:10.1007/BF00246995.
- ↑ 17.0 17.1 17.2 "Über Entwicklungen auf dem Gestagengebiet. 15.". General Assembly of the Japan Medical Congress, Tokyo. 1. 1959. pp. 697–706.
- ↑ "Formulation". https://www.google.com/patents/US6774122.
- ↑ 19.0 19.1 "Notes - Steroids. LXXVI. Synthesis of Long Chain Carboxylic Acid Esters of 17α-Hydroxyprogesterone". The Journal of Organic Chemistry 21 (2): 240–241. 1956. doi:10.1021/jo01108a601. ISSN 0022-3263.
- ↑ "Anti-gonadotropic steroids, inhibition of ovulation and mating". Methods in Hormone Research. 2. Elsevier. 1962. pp. 252–. ISBN 978-1-4832-7276-4. https://books.google.com/books?id=WS_LBAAAQBAJ&pg=PA252.
- ↑ "The duration of activity of the benziloyl hydrazones of testosterone-17-heptanoate, estrone-3-heptanoate and 17α-hydroxy-progesterone-17-heptanoate". Endocrinology 65 (3): 508–511. 1959. doi:10.1210/endo-65-3-508. ISSN 0013-7227. PMID 13828402.
- ↑ "OHPH". micromedexsolutions.com. https://www.micromedexsolutions.com/.
 |
