Chemistry:Trendione
From HandWiki
Short description: Chemical compound
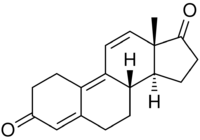 | |
| Clinical data | |
|---|---|
| Other names | RU-2065; Trenavar; Triendione; Estra-4,9,11-triene-3,17-dione |
| Drug class | Androgen; Anabolic steroid; Progestogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C18H20O2 |
| Molar mass | 268.356 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Trendione (developmental code name RU-2065; nickname Trenavar), also known as estra-4,9,11-triene-3,17-dione, is an androgen prohormone as well as metabolite of the anabolic steroid trenbolone.[1][2][3][4][5] Trendione is to trenbolone as androstenedione is to testosterone.[6] The compound is inactive itself, showing more than 100-fold lower affinity for the androgen and progesterone receptors than trenbolone.[7][8] It is a designer steroid and has been sold on the internet as a "nutritional supplement".[1] Trendione is listed in the United States Designer Anabolic Steroid Control Act of 2014.[1]
See also
- List of androgens/anabolic steroids
References
- ↑ 1.0 1.1 1.2 "Guerilla Warfare on the Pancreas? A Case of Acute Pancreatitis From a Supplement Known to Contain Anabolic-Androgenic Steroids". Mil Med 181 (10): e1395–e1397. October 2016. doi:10.7205/MILMED-D-15-00575. PMID 27753588.
- ↑ "Tissue selectivity and potential clinical applications of trenbolone (17beta-hydroxyestra-4,9,11-trien-3-one): A potent anabolic steroid with reduced androgenic and estrogenic activity". Steroids 75 (6): 377–89. June 2010. doi:10.1016/j.steroids.2010.01.019. PMID 20138077.
- ↑ "Metabolism of some anabolic agents: toxicological and analytical aspects". J. Chromatogr. 489 (1): 11–21. April 1989. doi:10.1016/s0378-4347(00)82880-7. PMID 2745641.
- ↑ "17β-Hydroxyestra-4,9,11-trien-3-one (trenbolone) exhibits tissue selective anabolic activity: effects on muscle, bone, adiposity, hemoglobin, and prostate". Am. J. Physiol. Endocrinol. Metab. 300 (4): E650–60. April 2011. doi:10.1152/ajpendo.00440.2010. PMID 21266670.
- ↑ "Disposition of 17 beta-trenbolone in humans". J. Chromatogr. 564 (2): 485–92. April 1991. doi:10.1016/0378-4347(91)80517-g. PMID 1874853.
- ↑ Advances in Agronomy. Elsevier. 13 April 2007. pp. 16–. ISBN 978-0-08-048819-6. https://books.google.com/books?id=ydh79BFwCnoC&pg=PA16.
- ↑ "Characterisation of the affinity of different anabolics and synthetic hormones to the human androgen receptor, human sex hormone binding globulin and to the bovine progestin receptor". APMIS 108 (12): 838–46. December 2000. doi:10.1111/j.1600-0463.2000.tb00007.x. PMID 11252818.
- ↑ "Steroid flexibility and receptor specificity". J. Steroid Biochem. 13 (1): 45–59. January 1980. doi:10.1016/0022-4731(80)90112-0. PMID 7382482.
 |
