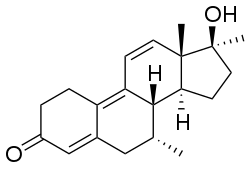
Chemistry:Dimethyltrienolone
 | |
| Clinical data | |
|---|---|
| Other names | RU-2420; 7α,17α-Dimethyltrenbolone; 7α,17α-Dimethyl-δ9,11-19-nortestosterone; 7α,17α-Dimethylestra-4,9,11-trien-17β-ol-3-one |
| Routes of administration | By mouth |
| Drug class | Androgen; Anabolic steroid; Progestogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C20H26O2 |
| Molar mass | 298.426 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Dimethyltrienolone (developmental code name RU-2420) is a synthetic, orally active, and extremely potent anabolic–androgenic steroid (AAS) and 17α-alkylated 19-nortestosterone (nandrolone) derivative which was never marketed for medical use.[1] It has among the highest known affinity of any AAS for the androgen (and progesterone) receptors,[2][3] and has been said to be perhaps the most potent AAS to have ever been developed.[1]
Pharmacology
Pharmacodynamics
Dimethyltrienolone is an extremely potent agonist of the androgen and progesterone receptors and hence AAS and progestogen.[1] In animal bioassays, it was shown to possess more than 100 times the anabolic and androgenic potency of the reference AAS methyltestosterone.[1] The drug is not a substrate for 5α-reductase and so is not potentiated or inactivated in so-called "androgenic" tissues like the prostate gland or skin.[1] It is also not a substrate for aromatase and so has no estrogenic activity.[1] Due to its lack of estrogenicity, dimethyltrienolone has no propensity for causing estrogenic side effects like gynecomastia.[1] Because of its C17α methyl group and very high resistance to hepatic metabolism, dimethyltrienolone is said to be exceedingly hepatotoxic.[1]
| Compound | Chemical name | PR | AR | ER | GR | MR | ||
|---|---|---|---|---|---|---|---|---|
| Testosterone | T | 1.0 | 100 | <0.1 | 0.17 | 0.9 | ||
| Nandrolone | 19-NT | 20 | 154 | <0.1 | 0.5 | 1.6 | ||
| Trenbolone | ∆9,11-19-NT | 74 | 197 | <0.1 | 2.9 | 1.33 | ||
| Trestolone | 7α-Me-19-NT | 50–75 | 100–125 | ? | <1 | ? | ||
| Normethandrone | 17α-Me-19-NT | 100 | 146 | <0.1 | 1.5 | 0.6 | ||
| Metribolone | ∆9,11-17α-Me-19-NT | 208 | 204 | <0.1 | 26 | 18 | ||
| Mibolerone | 7α,17α-DiMe-19-NT | 214 | 108 | <0.1 | 1.4 | 2.1 | ||
| Dimethyltrienolone | ∆9,11-7α,17α-DiMe-19-NT | 306 | 180 | 0.1 | 22 | 52 | ||
| Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, estradiol for the ER, DEXA for the GR, and aldosterone for the MR. | ||||||||
Chemistry
Dimethyltrienolone, also known as 7α,17α-dimethyl-δ9,11-19-nortestosterone or as 7α,17α-dimethylestra-4,9,11-trien-17β-ol-3-one, as well as 7α,17α-dimethyltrenbolone, is a synthetic estrane steroid and a 17α-alkylated derivative of nandrolone (19-nortestosterone).[1] It is the 7α,17α-dimethyl derivative of trenbolone and the 7α-methyl derivative of metribolone,[6] as well as the δ9,11 analogue of metribolone and the δ9,11, 17α-methylated derivative of trestolone.[1]
History
Dimethyltrienolone was first described in 1967.[1][7] It was never marketed for medical use.[1]
See also
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 William Llewellyn (2009). Anabolics. Molecular Nutrition Llc. pp. 212–214. ISBN 978-0967930473. https://books.google.com/books?id=afKLA-6wW0oC.
- ↑ "PRO_LIGAND: an approach to de novo molecular design. 2. Design of novel molecules from molecular field analysis (MFA) models and pharmacophores". Journal of Medicinal Chemistry 37 (23): 3994–4002. November 1994. doi:10.1021/jm00049a019. PMID 7966160.
- ↑ "A comparison of progestin and androgen receptor binding using the CoMFA technique". Journal of Computer-Aided Molecular Design 6 (6): 569–581. December 1992. doi:10.1007/bf00126215. PMID 1291626. Bibcode: 1992JCAMD...6..569L.
- ↑ "Steroid flexibility and receptor specificity". Journal of Steroid Biochemistry 13 (1): 45–59. January 1980. doi:10.1016/0022-4731(80)90112-0. PMID 7382482.
- ↑ "Towards the mapping of the progesterone and androgen receptors". Journal of Steroid Biochemistry 27 (1–3): 255–269. 1987. doi:10.1016/0022-4731(87)90317-7. PMID 3695484.
- ↑ "Antiprogestins". Actions of Progesterone on the Brain. Springer Science & Business Media. 6 December 2012. pp. 17–. ISBN 978-3-642-69728-9. https://books.google.com/books?id=CFEICQAAQBAJ&pg=PA17.
- ↑ Proceedings of the International Symposium on Drug Research, Montreal, Canada, June 12-14, 1967. Chemical Institute of Canada, Medical Chemistry Group, Montreal, Canada. 1967. p. 134. https://books.google.com/books?id=A69ZnQAACAAJ.
 |
