Chemistry:Norethisterone acetate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Primolut-Nor, Aygestin, Gestakadin, Milligynon, Monogest, Norlutate, Primolut N, SH-420, Sovel, Styptin, others |
| Other names | NETA; NETAc; Norethindrone acetate; SH-420; 17α-Ethynyl-19-nortestosterone 17β-acetate; 17α-Ethynylestra-4-en-17β-ol-3-one 17β-acetate |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a604034 |
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin; Progestogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H28O3 |
| Molar mass | 340.463 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Norethisterone acetate (NETA), also known as norethindrone acetate and sold under the brand name Primolut-Nor among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders.[1][2][3][4] The medication available in low-dose and high-dose formulations and is used alone or in combination with an estrogen.[5][4][6][7] It is ingested orally.[6]
Side effects of NETA include menstrual irregularities, headaches, nausea, breast tenderness, mood changes, acne, increased hair growth, and others.[6] NETA is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[1] It has weak androgenic and estrogenic activity and no other important hormonal activity.[1][8] The medication is a prodrug of norethisterone in the body.[9][10]
NETA was patented in 1957 and was introduced for medical use in 1964.[11][12] It is sometimes referred to as a "first-generation" progestin.[13][14] NETA is marketed widely throughout the world.[4] It is available as a generic medication.[15]
Medical uses
NETA is used as a hormonal contraceptive in combination with estrogen, in the treatment of gynecological disorders such as abnormal uterine bleeding, and as a component of menopausal hormone therapy for the treatment of menopausal symptoms.[4]
Available forms
NETA is available in the form of tablets for use by mouth both alone and in combination with estrogens including estradiol, estradiol valerate, and ethinylestradiol.[16][4] Transdermal patches providing a combination of 50 μg/day estradiol and 0.14 or 0.25 mg/day NETA are available under the brand names CombiPatch and Estalis.[16][4]
NETA was previously available for use by intramuscular injection in the form of ampoules containing 20 mg NETA, 5 mg estradiol benzoate, 8 mg estradiol valerate, and 180 mg testosterone enanthate in oil solution under the brand name Ablacton to suppress lactation in postpartum women.[17][18][19][20]
Contraindications
Side effects
Side effects of NETA include menstrual irregularities, headaches, nausea, breast tenderness, mood changes, acne, increased hair growth, and others.[6]
Overdose
Interactions
Pharmacology
Pharmacodynamics
NETA is a prodrug of norethisterone in the body.[9] Upon oral ingestion, it is rapidly converted into norethisterone by esterases during intestinal and first-pass hepatic metabolism.[10] Hence, as a prodrug of norethisterone, NETA has essentially the same effects, acting as a potent progestogen with additional weak androgenic and estrogenic activity (the latter via its metabolite ethinylestradiol).[1][8]
| Compound | Typea | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|---|
| Norethisterone | – | 67–75 | 15 | 0 | 0–1 | 0–3 | 16 | 0 |
| 5α-Dihydronorethisterone | Metabolite | 25 | 27 | 0 | 0 | ? | ? | ? |
| 3α,5α-Tetrahydronorethisterone | Metabolite | 1 | 0 | 0–1 | 0 | ? | ? | ? |
| 3α,5β-Tetrahydronorethisterone | Metabolite | ? | 0 | 0 | ? | ? | ? | ? |
| 3β,5α-Tetrahydronorethisterone | Metabolite | 1 | 0 | 0–8 | 0 | ? | ? | ? |
| Ethinylestradiol | Metabolite | 15–25 | 1–3 | 112 | 1–3 | 0 | 0.18 | 0 |
| Norethisterone acetate | Prodrug | 20 | 5 | 1 | 0 | 0 | ? | ? |
| Norethisterone enanthate | Prodrug | ? | ? | ? | ? | ? | ? | ? |
| Noretynodrel | Prodrug | 6 | 0 | 2 | 0 | 0 | 0 | 0 |
| Etynodiol | Prodrug | 1 | 0 | 11–18 | 0 | ? | ? | ? |
| Etynodiol diacetate | Prodrug | 1 | 0 | 0 | 0 | 0 | ? | ? |
| Lynestrenol | Prodrug | 1 | 1 | 3 | 0 | 0 | ? | ? |
| Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone for the AR, estradiol for the ER, dexamethasone for the GR, aldosterone]] for the MR, dihydrotestosterone for SHBG, and cortisol for CBG. Footnotes: a = Active]] or inactive metabolite, prodrug, or neither of norethisterone. Sources: See template. | ||||||||
Progestogenic effects
In terms of dosage equivalence, norethisterone and NETA are typically used at respective dosages of 0.35 mg/day and 0.6 mg/day as progestogen-only contraceptives, and at respective dosages of 0.5–1 mg/day and 1–1.5 mg/day in combination with ethinylestradiol in combined oral contraceptives.[8] Conversely, the two drugs have been used at about the same dosages in menopausal hormone therapy for the treatment of menopausal symptoms.[8] NETA is of about 12% higher molecular weight than norethisterone due to the presence of its C17β acetate ester.[2] Micronization of NETA has been found to increase its potency by several-fold in animals and women.[21][22][23][24] The endometrial transformation dosage of micronized NETA per cycle is 12 to 14 mg, whereas that for non-micronized NETA is 30 to 60 mg.[21]
Estrogenic effects
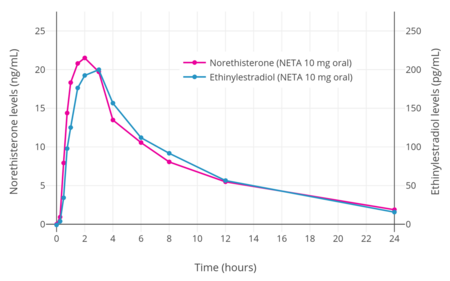
NETA metabolizes into ethinylestradiol at a rate of 0.20 to 0.33% across a dose range of 10 to 40 mg.[26][27] Peak levels of ethinylestradiol with a 10, 20, or 40 mg dose of NETA were 58, 178, and 231 pg/mL, respectively.[26][27] For comparison, a 30 to 40 μg dose of oral ethinylestradiol typically results in a peak ethinylestradiol level of 100 to 135 pg/mL.[27] As such, in terms of ethinylestradiol exposure, 10 to 20 mg NETA may be equivalent to 20 to 30 μg ethinylestradiol and 40 mg NETA may be similar to 50 μg ethinylestradiol.[27] In another study however, 5 mg NETA produced an equivalent of 28 μg ethinylestradiol (0.7% conversion rate) and 10 mg NETA produced an equivalent of 62 μg ethinylestradiol (1.0% conversion rate).[25][28] Due to its estrogenic activity via ethinylestradiol, high doses of NETA have been proposed for add-back in the treatment of endometriosis without estrogen supplementation.[26] Generation of ethinylestradiol with high doses of NETA may increase the risk of venous thromboembolism but may also decrease menstrual bleeding relative to progestogen exposure alone.[27][28]
Antigonadotropic effects
NETA has antigonadotropic effects via its progestogenic activity and can dose-dependently suppress gonadotropin and sex hormone levels in women and men.[1][29][30][31] The ovulation-inhibiting dose of NETA is about 0.5 mg/day in women.[1] In healthy young men, NETA alone at a dose of 5 to 10 mg/day orally for 2 weeks suppressed testosterone levels from ~527 ng/dL to ~231 ng/dL (–56%).[30]
Chemistry
NETA, also known as norethinyltestosterone acetate, as well as 17α-ethynyl-19-nortestosterone 17β-acetate or 17α-ethynylestra-4-en-17β-ol-3-one 17β-acetate, is a progestin, or synthetic progestogen, of the 19-nortestosterone group, and a synthetic estrane steroid.[2][5] It is the C17β acetate ester of norethisterone.[2][5] NETA is a derivative of testosterone with an ethynyl group at the C17α position, the methyl group at the C19 position removed, and an acetate ester attached at the C17β position.[2][5] In addition to testosterone, it is a combined derivative of nandrolone (19-nortestosterone) and ethisterone (17α-ethynyltestosterone).[2][5]
Synthesis
Chemical syntheses of NETA have been published.[32]
History
Schering AG filed for a patent for NETA in June 1957, and the patent was issued in December 1960.[11] The drug was first marketed, by Parke-Davis as Norlestrin in the United States , in March 1964.[11][12] This was a combination formulation of 2.5 mg NETA and 50 μg ethinylestradiol and was indicated as an oral contraceptive.[11][12] Other early brand names of NETA used in oral contraceptives included Minovlar and Anovlar.[11]
Society and culture
Generic names
Norethisterone acetate is the INN, BANM, and JAN of NETA while norethindrone acetate is its USAN and USP.[2][5][4]
Brand names
NETA is marketed under a variety of brand names throughout the world including Primolut-Nor (major), Aygestin (US), Gestakadin, Milligynon, Monogest, Norlutate (US, CA), Primolut N, SH-420 (UK), Sovel, and Styptin among others.[2][5][4]
Availability
United States
NETA is marketed in high-dose 5 mg oral tablets in the United States under the brand names Aygestin and Norlutate for the treatment of gynecological disorders.[33] In addition, it is available under a large number of brand names at much lower dosages (0.1 to 1 mg) in combination with estrogens such as ethinylestradiol and estradiol as a combined oral contraceptive and for use in menopausal hormone therapy for the treatment of menopausal symptoms.[7]
Research
NETA has been studied for use as a potential male hormonal contraceptive in combination with testosterone in men.[34]
See also
- Ethinylestradiol/norethisterone acetate
- Norethisterone enanthate
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric 8 (Suppl 1): 3–63. August 2005. doi:10.1080/13697130500148875. PMID 16112947. http://hormonebalance.org/images/documents/Kuhl%2005%20%20Pharm%20Estro%20Progest%20Climacteric_1313155660.pdf.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 886–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA886.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 750. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA750. Retrieved 30 May 2012.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 "Norethindrone Monograph for Professionals". https://www.drugs.com/ppa/norethindrone-acetate.html.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Cite error: Invalid
<ref>tag; no text was provided for refs namedIndexNominum2000 - ↑ 6.0 6.1 6.2 6.3 https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/018405s023lbl.pdf [bare URL PDF]
- ↑ 7.0 7.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedDrugs@FDA - ↑ 8.0 8.1 8.2 8.3 IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 417–. ISBN 978-92-832-1291-1. https://books.google.com/books?id=aGDU5xibtNgC&pg=PA417. "Norethisterone and its acetate and enanthate esters are progestogens that have weak estrogenic and androgenic properties."
- ↑ 9.0 9.1 Thomas L. Lemke; David A. Williams (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1316–. ISBN 978-0-7817-6879-5. https://books.google.com/books?id=R0W1ErpsQpkC&pg=PA1316.
- ↑ 10.0 10.1 "The hormonal profile of norethindrone acetate: rationale for add-back therapy with gonadotropin-releasing hormone agonists in women with endometriosis". Reprod Sci 19 (6): 563–71. 2012. doi:10.1177/1933719112438061. PMID 22457429.
- ↑ 11.0 11.1 11.2 11.3 11.4 Lara Marks (2010). Sexual Chemistry: A History of the Contraceptive Pill. Yale University Press. pp. 73–. ISBN 978-0-300-16791-7. https://books.google.com/books?id=_i-s4biQs7MC&pg=PA73.
- ↑ 12.0 12.1 12.2 Robert W. Blum (22 October 2013). Adolescent Health Care: Clinical Issues. Elsevier Science. pp. 216–. ISBN 978-1-4832-7738-7. https://books.google.com/books?id=36PpAgAAQBAJ&pg=PA216.
- ↑ Robert Anthony Hatcher; Anita L. Nelson, M.D. (2007). Contraceptive Technology. Ardent Media. pp. 195–. ISBN 978-1-59708-001-9. https://books.google.com/books?id=txh0LpjjhkoC&pg=PA195.
- ↑ Sulochana Gunasheela (14 March 2011). Practical Management of Gynecological Problems. JP Medical Ltd. pp. 31–. ISBN 978-93-5025-240-6. https://books.google.com/books?id=gZB-h_gqgS8C&pg=PA31.
- ↑ "Generic Aygestin Availability". https://www.drugs.com/availability/generic-aygestin.html.
- ↑ 16.0 16.1 James M. Rippe (15 March 2013). Lifestyle Medicine. CRC Press. pp. 280–. ISBN 978-1-4398-4544-8. https://books.google.com/books?id=9ibOBQAAQBAJ&pg=PA280.
- ↑ A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 696–. ISBN 978-3-642-96158-8. https://books.google.com/books?id=DAgJCAAAQBAJ&pg=PA696.
- ↑ F. G. Sulman (22 October 2013). Hypothalamic Control of Lactation: Monographs on Endocrinology. Elsevier Science. pp. 184–. ISBN 978-1-4831-9303-8. https://books.google.com/books?id=pMXaAgAAQBAJ&pg=PA184.
- ↑ Ufer, Joachim (1 January 1978) (in de). Hormontherapie in der Frauenheilkunde: Grundlagen und Praxis (5 ed.). de Gruyter. ISBN 978-3110066647. OCLC 924728827.
- ↑ Drugs. S. Karger. 1975. p. 128. https://books.google.com/books?id=r4BNAQAAIAAJ. "5.5.4 Oestradiol valerate + Benzoate/Testosterone Enanthate/Norethisterone Acetate (Ablacton). This product contains oestradiol benzoate 5mg, oestradiol valerate 8mg, norethisterone acetate 20mg and testosterone enanthate 180mg in a 1ml oily solution. It is injected intramuscularly."
- ↑ 21.0 21.1 J. Horsky; J. Presl (6 December 2012). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 313–. ISBN 978-94-009-8195-9. https://books.google.com/books?id=7IrpCAAAQBAJ&pg=PA313.
- ↑ Janet Brotherton (1976). Sex Hormone Pharmacology. Academic Press. p. 34. ISBN 978-0-12-137250-7. https://books.google.com/books?id=zt5sAAAAMAAJ.
- ↑ "Effect of particle size on biological activity of norethisterone acetate". Acta Physiol Lat Am 18 (4): 323–6. 1968. PMID 5753386.
- ↑ "Comparative cross-over pharmacokinetic study on two types of postcoital contraceptive tablets containing levonorgestrel". Contraception 41 (5): 557–67. May 1990. doi:10.1016/0010-7824(90)90064-3. PMID 2112080.
- ↑ 25.0 25.1 "In vivo conversion of norethisterone and norethisterone acetate to ethinyl etradiol in postmenopausal women". Contraception 56 (6): 379–85. 1997. doi:10.1016/s0010-7824(97)00174-1. PMID 9494772. "[...] it has been shown that the repeated oral administration of NET at doses of 0.5 to 3.0 mg to fertile women caused a dose related decrease in the serum levels of SHBG.24 It should be borne in mind that, besides its progestational activity, NET is also characterized by a marked androgenic partial activity, which has a suppressive effect on the synthesis of SHBG and therefore compensates the effects of an additional exposure to EE, on the liver.".
- ↑ 26.0 26.1 26.2 "Characteristics and metabolic effects of estrogen and progestins contained in oral contraceptive pills". Best Pract. Res. Clin. Endocrinol. Metab. 27 (1): 13–24. February 2013. doi:10.1016/j.beem.2012.09.004. PMID 23384742.
- ↑ 27.0 27.1 27.2 27.3 27.4 "Formation of ethinyl estradiol in women during treatment with norethindrone acetate". J. Clin. Endocrinol. Metab. 92 (6): 2205–7. June 2007. doi:10.1210/jc.2007-0044. PMID 17341557.
- ↑ 28.0 28.1 "Norethisterone Reduces Vaginal Bleeding Caused by Progesterone-Only Birth Control Pills". J Clin Med 11 (12): 3389. June 2022. doi:10.3390/jcm11123389. PMID 35743459.
- ↑ "Ovulation inhibition doses of progestins: a systematic review of the available literature and of marketed preparations worldwide". Contraception 84 (6): 549–57. December 2011. doi:10.1016/j.contraception.2011.04.009. PMID 22078182.
- ↑ 30.0 30.1 "Impact of various progestins with or without transdermal testosterone on gonadotropin levels for non-invasive hormonal male contraception: a randomized clinical trial". Andrology 5 (3): 516–526. May 2017. doi:10.1111/andr.12328. PMID 28189123.
- ↑ "Potential of norethisterone enanthate for male contraception: pharmacokinetics and suppression of pituitary and gonadal function". Clin Endocrinol (Oxf) 53 (3): 351–8. September 2000. doi:10.1046/j.1365-2265.2000.01097.x. PMID 10971453.
- ↑ Die Gestagene. Springer-Verlag. 27 November 2013. pp. 14. ISBN 978-3-642-99941-3. https://books.google.com/books?id=t8GpBgAAQBAJ&pg=PA14.
- ↑ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. http://www.accessdata.fda.gov/scripts/cder/daf/.
- ↑ "Clinical trials in male hormonal contraception". Contraception 82 (5): 457–70. 2010. doi:10.1016/j.contraception.2010.03.020. PMID 20933120. http://www.kup.at/kup/pdf/10172.pdf.
 |

