Chemistry:Melengestrol acetate
 | |
| Clinical data | |
|---|---|
| Trade names | Heifermax, MGA |
| Other names | MGA; MLA; MLGA; Melengesterol acetate; Methylsuperlutin; U-21240; BDH-1921; 17α-Acetoxy-16-methylene-6-methyl-6-dehydroprogesterone; 17α-Acetoxy-16-methylene-6-methylpregna-4,6-diene-3,20-dione |
| Drug class | Progestogen; Progestin; Progestogen ester |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C25H32O4 |
| Molar mass | 396.527 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Melengestrol acetate (MLGA), sold under the brand names Heifermax and MGA among others, is a progestin medication which is used in animal reproduction.[1][2] It is not approved for use in humans, and is instead used as an implantable contraceptive for captive animals in zoos and other refuges,[3] and is also used as a feed additive to promote growth in cattle, a purpose it is licensed for in the United States and Canada .[4]
Uses
Animal reproduction
MLGA is used in animal reproduction.[3][4]
Pharmacology
Pharmacodynamics
MLGA is a progestogen, and hence is an agonist of the progesterone receptor.[5] It has been found to possess 73% of the affinity of progesterone for the progesterone receptor in rhesus monkey uterus.[5]
Chemistry
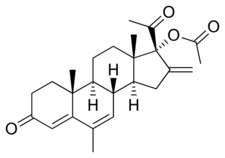
MLGA, also known as 17α-acetoxy-16-methylene-6-dehydro-6-methylprogesterone or as 17α-acetoxy-16-methylene-6-methylpregna-4,6-diene-3,20-dione, is a synthetic pregnane steroid and a derivative of progesterone.[1][2] It is specifically a derivative of 17α-hydroxyprogesterone with a methyl group at the C6 position, a methylene group at the C16 position, a double bond between the C6 and C7 positions, and an acetate ester at the C17α position.[1][2] As such, it is also a derivative of 16-methylene-17α-hydroxyprogesterone acetate. MLGA is the acetate ester of melengestrol, which in contrast, has never been marketed.[1] Analogues of MLGA include other 17α-hydroxyprogesterone derivatives such as chlormadinone acetate, chlormethenmadinone acetate, cyproterone acetate, delmadinone acetate, hydroxyprogesterone caproate, medroxyprogesterone acetate, megestrol acetate, methenmadinone acetate, and osaterone acetate.[1] The only structural difference between MLGA and megestrol acetate is the presence of the C16 methylene group in the former.[1]
Society and culture
Generic names
Melengestrol acetate is the generic name of the drug and its USAN and USP.[1][2] Melengestrol is the INN and BAN of the unesterified free alcohol form.[1][2]
Brand names
MLGA is marketed under the brand names Heifermax and MGA among others.[1][2]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 764–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA764.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "Melengestrol". Drugs.com. https://www.drugs.com/international/melengestrol.html.
- ↑ 3.0 3.1 "Chapter 34: Contraception as a Management Tool for Controlling Surplus Animals". Wild Mammals in Captivity: Principles and Techniques for Zoo Management (2nd ed.). Chicago, IL: University of Chicago Press. 2010. pp. 469–482. ISBN 9780226440118. https://books.google.com/books?id=a1vev5hf7o8C&pg=PA469. Retrieved 17 March 2016.
- ↑ 4.0 4.1 "Chapter 4: Current Analytical Methods Used for the Detection of Hormone Residues". Analyses for Hormonal Substances in Food-Producing Animals. Royal Society of Chemistry. 26 November 2009. p. 139. doi:10.1039/9781849730723-00129. ISBN 978-0-85404-198-5. https://books.google.com/books?id=9V-DbnzZiiMC&pg=PA139. Retrieved 27 May 2012.
- ↑ 5.0 5.1 "A specific progesterone receptor of myometrial cytosol from the rhesus monkey". Journal of Steroid Biochemistry 8 (2): 157–160. February 1977. doi:10.1016/0022-4731(77)90040-1. PMID 405534.
 |
