Chemistry:Gestadienol acetate
 | |
| Clinical data | |
|---|---|
| Other names | CIBA-31458-Ba; CIBA-31458; Norhydroxy-δ6-progesterone acetate; 6-Dehydro-17α-acetoxy-19-norprogesterone; 17α-Acetoxy-19-norpregn-4,6-diene-3,20-dione |
| Routes of administration | By mouth[1][2] |
| Drug class | Progestogen; Progestogen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C22H28O4 |
| Molar mass | 356.462 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Gestadienol acetate (developmental code name CIBA-31458-Ba or CIBA-31458) an orally active progestin which was described in the literature in 1967 and was never marketed.[3][1][2][4][5] It has no androgenic or estrogenic effects.[6] The effects of gestadienol acetate on the endometrium and its general pharmacology were studied in a clinical trial in women.[2][5] It has also been studied in a clinical trial for benign prostatic hyperplasia in men, but was ineffective.[6]
Chemistry
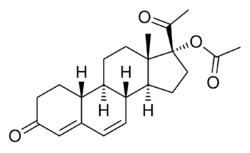
Gestadienol acetate, also known as norhydroxy-δ6-progesterone acetate, 6-dehydro-17α-hydroxy-19-norprogesterone 17α-acetate, or 17α-hydroxy-19-norpregn-4,6-diene-3,20-dione 17α-acetate, is a synthetic norpregnane steroid and a derivative of progesterone.[3] It is specifically a combined derivative of 17α-hydroxyprogesterone and 19-norprogesterone, or of gestronol (17α-hydroxy-19-norprogesterone), with an acetate ester at the C17α position and a double bond between the C6 and C7 positions.[3] Gestadienol acetate is the C17α acetate ester of gestadienol.[3] Analogues of gestadienol acetate include algestone acetophenide (dihydroxyprogesterone acetophenide), demegestone, gestonorone caproate (norhydroxyprogesterone caproate), hydroxyprogesterone acetate, hydroxyprogesterone caproate, nomegestrol acetate, norgestomet, and segesterone acetate (nestorone).[3]
References
- ↑ 1.0 1.1 "Biological Properties of a New Progesterone Analogue". European Journal of Endocrinology 56 (1 Suppl): S143. 1967. doi:10.1530/acta.0.056S143. ISSN 0804-4643.
- ↑ 2.0 2.1 2.2 "Effect of the new progestogen, CIBA 31458 on the human endometrium.". Journal of Reproduction and Fertility (Cambridge, England) 14 (3): 530. January 1967. http://www.reproduction-online.org/content/14/3/523.full.pdf.
- ↑ 3.0 3.1 3.2 3.3 3.4 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. p. 659. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA659.
- ↑ "The contraceptive progestagens". Chemical Reviews 70 (6): 713–726. December 1970. doi:10.1021/cr60268a004. PMID 4098492.
- ↑ 5.0 5.1 "Between basic and applied research: Ciba's involvement in steroids in the 1950s and 1960s". Steroids 61 (8): 492–503. August 1996. doi:10.1016/0039-128x(96)00040-2. PMID 8870170.
- ↑ 6.0 6.1 "Benign prostatic hypertrophy treated with a gestagen. A double-blind clinical trial with randomized allocation". Scandinavian Journal of Urology and Nephrology 6 (Suppl 157): 157–163. 1972. doi:10.3109/00365597209133659. PMID 4118753.
 |

